Translate this page into:
Electroencephalograph and optic nerve sheath diameter: Comparative usefulness in prediction of hepatic encephalopathy outcome in children
*Corresponding author: Roshan Koul, Department of Neurology, Institute of Liver and Biliary Sciences, New Delhi, India. koulroshan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Koul R, Alam S. Electroencephalograph and optic nerve sheath diameter: Comparative usefulness in prediction of hepatic encephalopathy outcome in children. J Neurosci Rural Pract. doi: 10.25259/JNRP_571_2023
Abstract
This study was carried out to compare the usefulness of electroencephalograph (EEG) and optic nerve sheath diameter (ONSD) in the prediction of outcomes in children undergoing management of hepatic encephalopathy (HE) in the intensive care unit (ICU). Twelve children in the age group 8–14 years with HE and raised intracranial pressure (ICP) were reviewed retrospectively for the prognostication value of EEG and ONSD in their management. The study period was from January 2019 to December 2021. The children were selected consecutively as they were admitted to the ICU. Children admitted to the ICU for the management of acute liver failure, HE, and raised ICP were followed up until recovery or death. Serial recordings of EEG and ONSD were done in twelve children during the management of their critical illness period. ONSD is a physical parameter based on cerebrospinal fluid dynamics while EEG is an electrophysiological measure revealing brain activity. Out of 12 children, ONSD normalized in 4, among these four children, three survived and one expired. Nine children had grade 3 and 4 encephalopathy patterns in their EEGs, and no one survived while three children with EEG grade 1 and 2 encephalopathy, all survived. ONSD values and EEG grades most of the time go together; however, EEG predicted more accurately the survival or death. In addition, seizures were seen in three children. EEG and ONSD are sensitive and useful non-invasive techniques in the prognosis and management of HE, EEG may have an edge over ONSD in predicting outcomes.
Keywords
Hepatic encephalopathy
Raised intracranial pressure
Optic nerve sheath diameter
Electroencephalograph
Outcome
INTRODUCTION
Several noninvasive measures are used in monitoring the brain function of hepatic encephalopathy (HE) patients with raised intracranial pressure (ICP) and acute liver failure (ALF) in the intensive care unit (ICU).[1] These non-invasive measures help in the day-to-day management of raised ICP.[1] Cerebral edema and raised ICP result in high mortality in ALF patients.[2] Elevated ammonia levels are not the only cause of their raised ICP; they are also a predictor of the events to come.[3,4] More than one type of non-invasive monitors are used in ICU settings during the management of ALF. The superiority of one over another is difficult to compare. Electroencephalography (EEG) is one of the non-invasive measures used in monitoring brain activity.[1,4-6] A simple ultrasound technique is used to measure optic nerve sheath diameter (ONSD). In this study, EEG and ONSD were compared to know their prognostic value for the outcome of HE in ALF children.
MATERIALS AND METHODS
This is a case series. From January 2019 to December 2021, children aged below 15 years admitted to the liver ICU (LICU) for the management of ALF associated with raised ICP were evaluated. The children were selected consecutively as they were admitted. All children below 15-year-old age group were included. All these children were intubated and on propofol, atracurium, and fentanyl. Only those children who had both ONSD monitoring and EEG were included in the study. ONSD was measured several times per day (usually 6 hourly) and before and after therapy for raised ICP until the pressure normalized or the child did not survive. A mean value of several readings of ONSD per day was finally selected for the assessment in this study. Usually, a single DM hepatology resident posted in the ICU would monitor ONSD every 6 h. However, same-observer and inter-observer errors were still possible. A mean of several 24-h readings was considered as one reading of that day.
Intravenous mannitol and 3% saline were used to lower the ICP. 20% mannitol (1 G/kg) was used intermittently in addition to 3% saline based on the ICP monitoring based on ONSD. 3% saline was used to target a serum sodium level in the 145–150 mEq/L range. The formula is based on 0.6 × body weight × difference between 145 and current serum sodium level. This difference up to a maximum of 10 mEq was given over 24-h duration as a correction. 1 mL of 3% saline is equal to 0.5 mEq of sodium.
The EEG was recorded to monitor the grade of encephalopathy. A Nihon Kohden portable machine (USA), especially used in ICUs, was used to record the EEGs in the ICU. Other appropriate precautions were taken to avoid electrical and medical equipment artifacts. At least two or more EEGs were recorded in a given patient. The EEG grading was based on the established criteria with a little modification,[6,7] as below:
Normal
Mild slowing, theta waves, intermittent alpha
More slowing, a delta background, and occasional T waves
Slow waves in the delta range, T waves, periodicity, suppression burst
Low amplitude/electrocerebral silence
Seizures/status/focal/generalized
Brain imaging was not routinely done. Only two children had imaging of the brain (one with computerized tomography and the second with magnetic resonance imaging). Hospital ethical committee approval for case reports and surveys is not required. The diagnostic accuracy test was used for the analysis of sensitivity and specificity.
RESULTS
In a 3-year period, twelve children (6M: 6F) under 15 years of age, who had both ONSD and EEG recordings in LICU, were seen. All were intubated and had raised ICP. The age ranged between 8 and 14 years, with a mean of 11.57 years. Hospitalization days ranged between 1 and 18 days, with a mean of 6.3 days. Nine of the 12 (75%) children died. The underlying etiology was hepatitis A in 6, indeterminate in 3, drug-induced in 1, hepatitis E in 1, and dengue in 2. (One patient had both hepatitis A and dengue) [Table 1]. The EEG patterns of stages 2, 3, 4, and 5 are given in Figure 1. Four children had ONSD below 4.9, out of which three survived. On the other hand, children with EEG grade 2 (HE) or below survived. None of the children with EEG grade 3 or above survived. The correlation between ONSD and EEG in relation to mortality is shown in Figure 2. On the diagnostic accuracy test, EEG sensitivity and specificity were 100%, while ONSD was 88.89% and 66.67%. Positive predictive value and net present value were 100% with EEG and 88.89% and 66.67%, respectively, with ONSD. Three children had seizures during the ICU treatment time, and one of them survived.
| Serial number | Age in years and sex | Etiology | Brain imaging | ONSD in mm | Outcome | EEG grade |
|---|---|---|---|---|---|---|
| 1 | 8M | Hepatitis E | No | 5.33 | Death | 4 |
| 2 | 13M | Indeterminate | No | 5.57 | Death | 4 |
| 3 | 12F | Indeterminate | CT^ICT | 4.03 | Discharged | 2 |
| 4 | 13F | Hepatitis A | No | 5.93 | Death/ELC | 4 |
| 5 | 10M | Indeterminate | No | 4.91 | Death | 4 |
| 6 | 11F | DILI | CT^ICT | 4.05 | Discharged | 2 |
| 7 | 12F | Hepatitis A | No | 5.37 | Death | 4/5 |
| 8 | 8.8M | Hepatitis A | MRI right frontal granuloma | 4.78 | Discharged | 2 |
| 9 | 14M | Dengue | No | 4.23 | Death | 3/4 |
| 10 | 11F | Hepatitis A | No | 4.94 | Death | 4/S |
| 11 | 14M | Hepatitis A | No | 4.64 | Death | 3/4 |
| 12 | 11M | Hepatitis A and dengue | No | 5.85 | Death | 4 |
Table shows various parameters in the 12 children with ALF with raised intracranial tensions, M: Male, F: Female, DILI: drug-induced liver injury, CT: Computerized tomography, ICT: Increased intracranial tension, MRI: Magnetic resonance imaging, ONSD: Optic nerve sheath diameter, ELC: End of life care, number in EEG grades indicates severity of hepatic encephalopathy, S: Seizures, EEG: Electroencephalograph
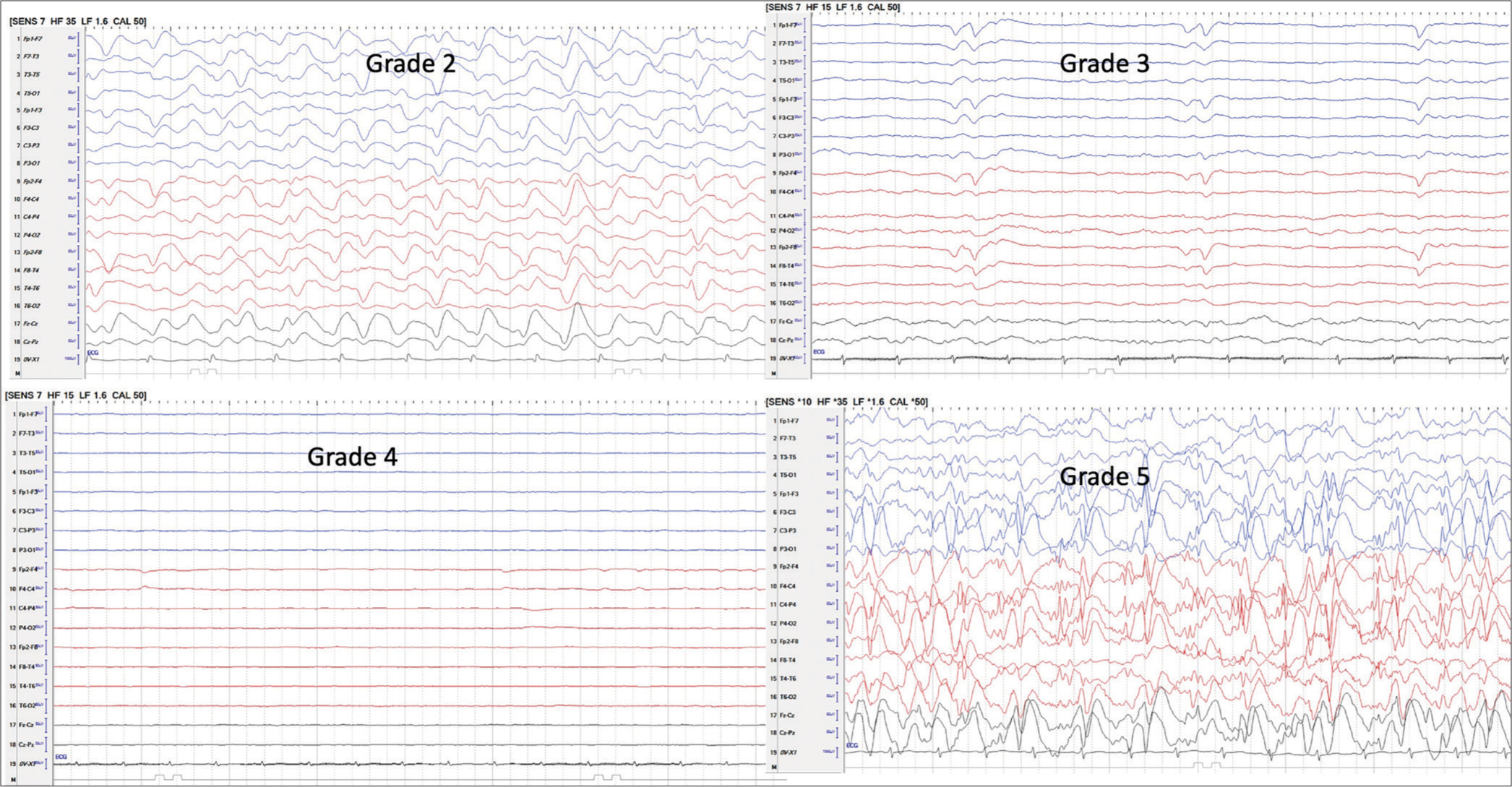
- Shows grades of electroencephalograph. Grade 2: More slowing, delta background, occasional T waves, Grade 3: Slow waves in delta range, T waves, periodicity, suppression burst, Grade 4: Low amplitude/electro cerebral silence, Grade 5: Seizures/status/focal/generalized, grade 1 and normal - grade 0 not shown.
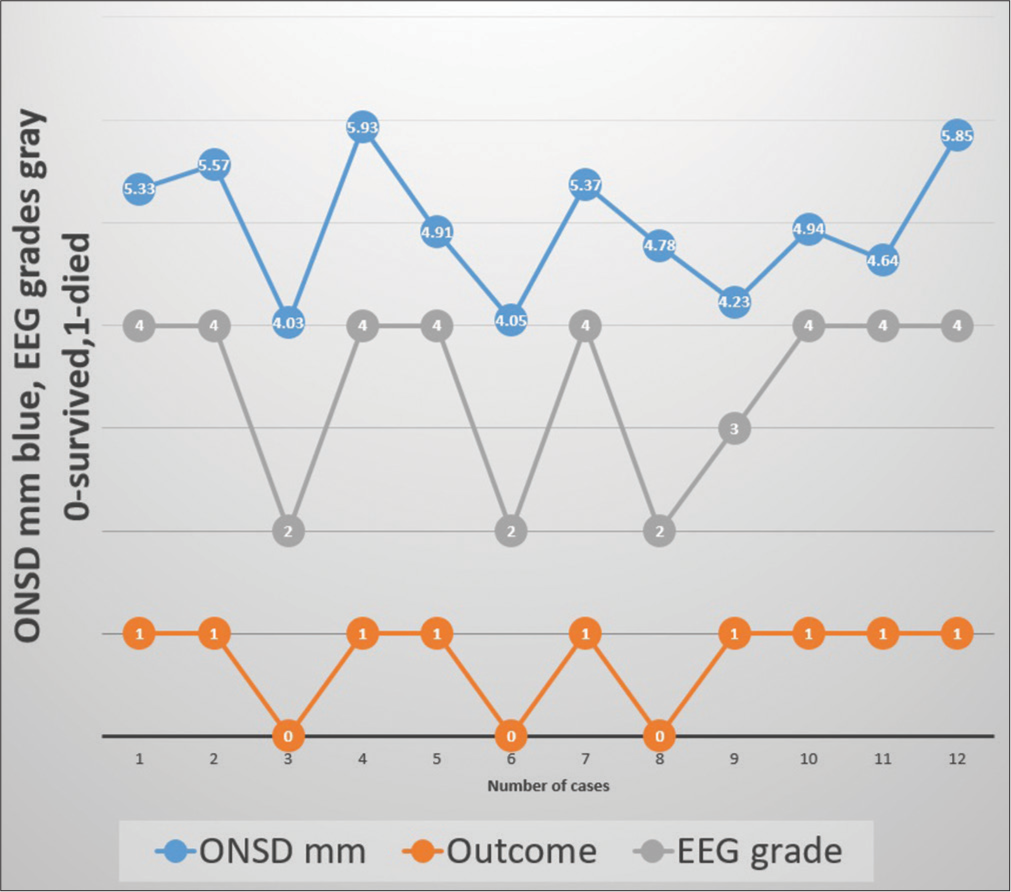
- Shows correlation between optic nerve sheath diameter (ONSD), electroencephalograph (EEG) and outcome.
DISCUSSION
A lot of studies have reported on ONSD and EEG separately in the management of pediatric-onset ALF, HE, and associated raised ICP. Raised ICP is one of the main causes of brain injury and mortality in ALF.[8] Several factors, including elevated ammonia, have been considered to be the cause of encephalopathy and raised ICP.[3,4,8] Nine out of twelve children died in this series. Mortality in the range of 30–85% has been reported in ALF and HE. Pediatric ALF (PALF) survival has improved with early management of ICP and liver transplantation.[9,10] The outcome of ALF and HE is based on intensive management of ALF and raised ICP.[9] Clinical, invasive, and several non-invasive tests have been used in monitoring ICP. Invasive ICP monitoring is rarely done in PALF in view of the associated severe coagulopathy.[11] Several noninvasive techniques such as pupillometry, jugular bulb oxygen saturation, trance cranial Doppler, EEG, brain imaging, near infrared spectroscopy, and ONSD have been used to monitor ICP.[1] 20% mannitol (1 G/kg) was used intermittently in addition to 3% saline based on the ICP monitoring based on ONSD.[12] ONSD is one of the tools used to monitor ICP daily.[12-14] The ONSD is one of the quantitative tests that help measure the ICP. It can be performed frequently and is usually done before and after therapy for raised ICP. A normal diameter of ONSD is usually below 4.5 mm.[12] In this institute, a previous study on ONSD found that 4.55 mm in diameter was the upper limit of normal.[12] ONSD persisting more than 4.6 mm by 24 h of management of ICP predicted a poor outcome with sensitivity and specificity of 83.3% and 72.7%, respectively.[12]
ONSD is a physical parameter and does not indicate the function of the underlying brain. On the other hand, EEG monitors brain electrophysiological activity and helps in diagnosing grades of encephalopathy corresponding to its severity.[6] An EEG has been used in HE monitoring since 1954.[15] The EEG not only helps in grading HE, but it also helps in excluding conditions like seizures and encephalitis, which may masquerade as HE in patients with liver disease.[5] The EEG is a better indicator of the prognosis compared to the ONSD as it indirectly indicates brain functioning.[5,6] It was seen in our present study that when ONSD normalized, but EEG grades were 3 or 4 [Figure 1], indicating severe encephalopathy, none survived. Most of the time, grades of HE are described in relation to chronic liver disease with liver failure. In these patients, raised ICP features on the EEG are not usually seen. However, the rapid rise in ICP due to brain edema modifies the background and pattern of the EEG in ALF patients.[6] The effect of the raised ICP masks the triphasic waves, which are frequently seen in the chronic liver disease associated with HE.[6]
Nine out of 12 (75%) of our ALF children did not survive. Several factors could be the cause of this high mortality. Arriving at the late stage of ALF and associated raised ICP in all of them. In addition, the fact that no donor is available for a liver transplant within a short period of hospitalization could be another factor. Mortality in PALF is related to the underlying etiology, availability of ICU care, liver transplant facility, and region of the world.[16,17] Prior to the availability of liver transplants and high-standard ICU care, there was 85% mortality.[16] Mortality has been brought down with advanced ICU care and liver transplantation.[17]
CONCLUSION
Our present study suggests EEG and ONSD complement each other in the management and prognosis of HE. However, EEG predicts a more accurate prognosis than ONSD. The limitations of our study are that the number of cases is small. In the future, a prospective study on continuous EEG monitoring in PALF is planned. This will help in correlating EEG grade fluctuations in relation to the treatment of ICP and ONSD.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Neurological monitoring in acute liver failure. CONCISE REVIEWS | Hepatology. 2019;70:1830-1835.
- [CrossRef] [Google Scholar]
- Neurologic manifestations of acute liver failure. Handb Clin Neurol. 2014;120:645-59.
- [CrossRef] [Google Scholar]
- Fasting blood ammonia predicts risk and frequency of hepatic encephalopathy Episodes in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14:903-6.
- [CrossRef] [Google Scholar]
- Ammonia level and mortality in acute liver failure: A single-center experience. Ann Transplant. 2016;21:479-83.
- [CrossRef] [Google Scholar]
- Continuous electroencephalography (cEEG) changes precede clinical changes in a case of progressive cerebral edema. Neurocrit Care. 2013;18:261-5.
- [CrossRef] [Google Scholar]
- Role of EEG in predicting outcome of hepatic encephalopathy patients. Neurodiagn J. 2020;60:272-88.
- [CrossRef] [Google Scholar]
- Current diagnosis and classification of hepatic encephalopathy. J Clin Exp Hepatol. 2018;8:432-7.
- [CrossRef] [Google Scholar]
- Encephalopathy and cerebral edema in the setting of acute liver failure: Pathogenesis and management. Neurocrit Care. 2008;9:97-102.
- [CrossRef] [Google Scholar]
- EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-81.
- [CrossRef] [Google Scholar]
- Complications and use of intracranial pressure monitoring in patients with acute liver failure and severe encephalopathy. Liver Transpl. 2005;11:1581-9.
- [CrossRef] [Google Scholar]
- Dynamic optic nerve sheath (ONSD) guided management of raised intracranial pressure in pediatric acute liver failure. Hepatol Int. 2021;15:502-9.
- [CrossRef] [Google Scholar]
- Ultrasound non-invasive intracranial pressure assessment in paediatric neurocritical care: A pilot study. Childs Nerv Syst. 2020;36:117-24.
- [CrossRef] [Google Scholar]
- Optic nerve ultrasonography in monitoring treatment efficacy in pediatric idiopathic intracranial hypertension. Child Syst. 2020;36:1425-33.
- [CrossRef] [Google Scholar]
- Hepatic coma: The electroencephalographic pattern. J Clin Invest. 1955;34:790-9.
- [CrossRef] [Google Scholar]
- Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652-8.
- [CrossRef] [Google Scholar]
- Outcomes of children with and without hepatic encephalopathy from the pediatric acute liver failure study group. J Pediatr Gastroenterol Nutr. 2016;63:357-64.
- [CrossRef] [Google Scholar]






