Translate this page into:
Equipotency of lacosamide to levetiracetam in new onset focal epilepsy: A randomized controlled trial
*Corresponding author: Sanjeev Kumar Bhoi, Department of Neurology, All India Institute of Medical Sciences, Bhubaneswar, Odisha, India. sanjeev_bhoi@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jaiswal BK, Bhoi SK, Jha M, Samal P, Porey C. Equipotency of lacosamide to levetiracetam in new onset focal epilepsy: A randomized controlled trial. J Neurosci Rural Pract 2023;14:622-8.
Abstract
Objectives:
Levetiracetam (LEV) is a well-established broad spectrum antiseizure medication (ASM) effective in focal, generalized, and myoclonic seizures whereas lacosamide (LCM) is a comparatively newer ASM currently approved only as an add-on agent in focal seizures. The aim of the study was to assess the efficacy and the tolerability of oral LCM as monotherapy in adult people with epilepsy (PWE) with new onset focal onset epilepsy compared with those receiving LEV.
Materials and Methods:
In this open-label single-center non-inferiority trial, PWE aged between 16 and 65 years suffering from new onset focal seizures, with or without secondary generalization were put on LCM monotherapy or LEV monotherapy. Data regarding demographic characteristics, seizure type and etiology, LCM and LEV daily dose, seizure frequency at baseline and at 6 months of follow-up, and seizure freedom rates were recorded.
Results:
Thirty-five PWE on LCM (24 males), their mean age: 38.20 ± 16.62 years and 35 PWE on LEV (25 males, mean age: 38.91 ± 17.13 years) were enrolled. The most common type of seizure observed was focal to bilateral tonic-clonic seizure >70% followed by focal impaired awareness seizure and focal awareness seizure. Structural epilepsy was found in 21 among LCM group and 22 of LEV group. In the LCM group, the seizure frequency decreased from 3.33 ± 1.88 to 0.85 ± 1.09 (P = 0.001) at 6 months and from 3.61 ± 3.12 to 0.94 ± 1.24 (P = 0.001) in LEV group, intergroup difference (P = 0.74). At 6-month follow-up period, 78.9% in LCM arm and 87.9% in the LEV arm had experienced a 50% of reduction in seizure frequency while seizure freedom was attained in 43.3% of PWE in both the arms (P = 1). The most common treatment emergent adverse effects in the LCM group were fatiguability, dyspepsia, headache, and dizziness, while in the LEV group; somnolence and behavioral abnormality.
Conclusion:
Treatment with LCM met the non-inferiority criteria when compared with LEV. Therefore, it might be useful as first-line monotherapy for adults with newly diagnosed focal epilepsy.
Keywords
Focal seizure
Non-inferiority
Randomized controlled trial
Side effect
Follow-up
INTRODUCTION
Epilepsy is one of the most common neurological disorder affecting up to 2% of the population worldwide.[1] The goal of pharmacological therapy with antiseizure medications (ASMs) is to reduce the frequency of seizures and achieve a seizure-free state with minimal side effects.[2] Levetiracetam (LEV) is a broad spectrum ASM that binds to synaptic vesicle SV2A preventing neurotransmitter release and is effective against focal and generalized seizures with a high therapeutic index and good tolerability.[3] Lacosamide (LCM), the R-enantiomer of 2-acetamido-Nbenzyl-3-methoxypropionamide, is a novel functionalized amino acid having dual action-enhancement of sodium-channel slow inactivation and modulation of collapsing response mediator protein-2.[4] It is a relatively newer approved ASM effective and well-tolerated as adjunctive therapy for adult PWE with uncontrolled partial-onset seizures.[2,4,5] While ASM monotherapy remains the mainstay of initial treatment in new onset focal seizures, the choice of ASM should be personalized based on the epileptic symptoms, comorbidities, medical history, age, childbearing potential, drug tolerance profile, and potential drug-drug interactions in the individual PWE. LEV alongside carbamazepine and phenytoin have Level A evidence as initial monotherapy for adults with partial onset seizures.[6] Although the efficacy and safety profile of LCM has been established in many conducted trials, mostly as an adjunctive therapy[1,2,4,7,8] and monotherapy in few.[5,9-11] None however compared LEV versus LCM monotherapy in focal seizures. To the best of our knowledge till date, only one study has retrospectively compared the two in a restricted population of elderly individuals.[3]
Objectives
Primary objective
The primary objective of the study was to assess decrease in seizure frequency with LCM monotherapy compared with LEV monotherapy over 6 months of follow-up period in patients of new onset focal epilepsy with or without secondary generalization.
Secondary objective
The secondary objective of the study was to assess the side effect profile of both the drugs.
MATERIALS AND METHODS
Study design
This was a single-center, tertiary care, hospital-based, prospective, randomized, open label, non-inferiority, clinical trial that evaluated the efficacy and safety of oral LCM and LEV in new onset focal epilepsy. PWE were enrolled from neurology outpatient department and emergency services. The trial was approved by the Institute Ethics Committee (IEC/AIIMS BBSR/PG Thesis/2019-20/68). The sample size was calculated with 28 subjects per group to achieve a power of 80%, to detect a difference of three mean monthly seizure reduction between the study groups with a significant level of <0.05 using two-sided two sample t-test. Considering attrition rate of approximately 15%, 35 subjects were included in each group. Considering a success rate of 50% in LEV group and 80% in LCM group and a calculated non-inferiority margin of −0.5 with an estimated one sided 95% confidence interval (CI) or a 90% two-sided confidence interval.
Inclusion criteria
The following criteria were included in the study:
(a) Age group 16 years or more, (b) Newly detected focal epilepsy with or without secondary generalization (clinical, historical, or electroencephalographic [EEG] findings suggestive of focal seizure) as per ILAE 2017 classification,[12] and (c) PWE taking seizure treatment were eligible to participate provided that treatment had lasted for 2 weeks or less and had ended at least 3 days before randomization.
Exclusion criteria
The following criteria were excluded from the study:
(a) Pregnant women, (b) PWE with current or previous seizure clusters or seizure types other than focal epilepsy, (c) syndromic or non-epileptic seizures, (d) atrioventricular block or any relevant echocardiographic abnormalities, (e) treatment with any drug that might influence LEV or LCM metabolism, and (f) severe skin reactions
Data collection
All the cases were reviewed and pertinent data (clinical history, number of monthly seizures in each group, family history, review of previous medication chart, general and systemic examination, blood biochemistry, video EEG (vEEG), non-contrast computed tomography (CT), and magnetic resonance imaging [MRI] brain) were obtained and recorded as per standard care of treatment.
Routine vEEG recordings were performed preferably within 24 h of the index seizure or after stabilization. The recordings were acquired with digital EEG systems (Nicolet V 32) using 32 channels according to the international 10–20 system.
All study participants underwent neuroimaging using both CT scan and 1.5 Tesla MRI to delineate the epileptogenic focus. MRI was acquired in 61 PWE (87%). Failure to undergo MRI included non-compliance (n = 4), non-MRI compatible implant (n = 2), intellectual disability (n = 1), and claustrophobia (n = 2).
Randomization, treatment, and follow-up
Total 70 PWE were randomized based on computer generated randomization to receive LCM (n = 35, Group 1) and LEV (n = 35, Group 2). Four PWE were excluded at 1-month follow-up. Group 1 PWE received LCM of 50 mg twice daily with an escalated maximum dose 400 mg/day within 15–30 days depending on seizure control. Similarly Group 2 PWE received LEV 500 mg twice daily and maximum dose up to 4 g/day within 15–30 days. No further modification was allowed thereafter.
Two PWE on LCM arm developed status epilepticus and were managed with multiple ASMs including trial drug. Two PWE in LEV arm were lost to follow-up within 1 month of maintenance therapy. Sixty-six PWE completed 6-month follow-up and were analyzed per protocol [Figure 1a and b].
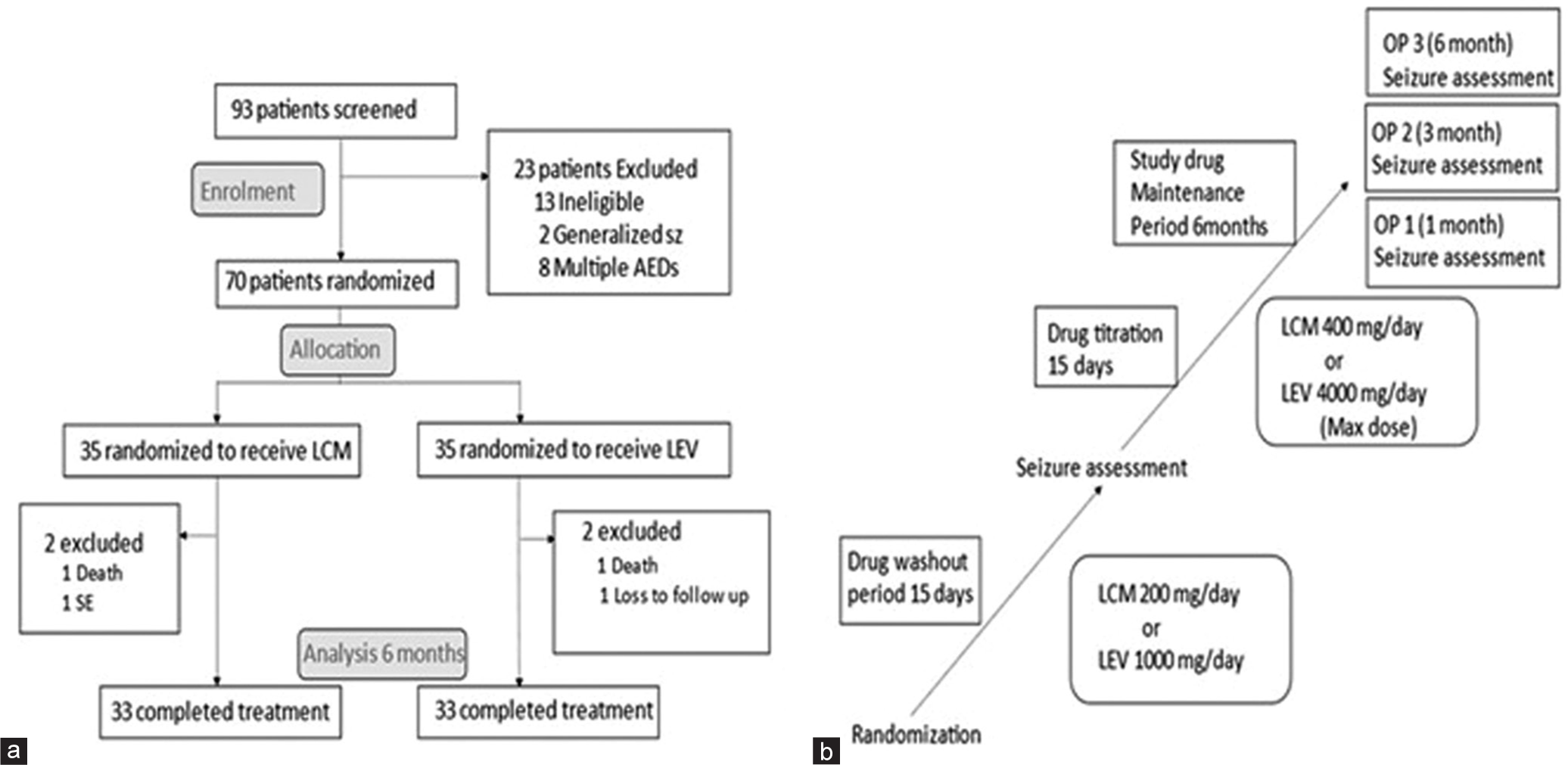
- (a) Flowchart showing consort statement and (b) Trial design. OP: Observation period, LCM: Lacosamide, LEV: Levetiracetam.
All PWE were followed up at outpatient department physically or teleconsultation platform for next 6 months during the COVID-19 pandemic. PWE were asked to maintain seizure or adverse effect diary and prioritized on emergency basis in any acute emergency. A second line ASM clobazam was added in four PWE in LCM group and two PWE in LEV group after development of second seizure episode. One subject underwent surgical removal of tumor in LEV group without recurrence of seizure.
Outcome
(i) Clinical efficacy between LCM and LEV monotherapy, (ii) >50% of baseline reduction in seizure frequency at 6-month follow-up, (iii) >100% seizure reduction or seizure freedom, and (iv) adverse events of both the trial drugs.
Statistical analysis
All data were entered in a predesigned proforma. Interpretation and analysis of obtained results were carried out using tests of significance. Statistical analysis was done on SPSS version 23 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism for windows, Version 8 (GraphPad software Inc., Sandiego, CA, USA). Qualitative data were analyzed by non-parametric tests and for quantitative data parametric tests were performed. P < 0.05 was considered statistically significant.
RESULTS
Demographic parameters
The present study had a total of 70 PWE with LCM and LEV group bearing 35 PWE each. Thirty-five PWE on LCM (24 males, 11 females, mean age: 38.20 ± 16.62, range: 16–65 years) and 35 PWE on LEV (27 males, eight females, mean age: 38.91 ± 17.13, range: 16–65 years) were enrolled in this trial. The mean duration of illness was 12.25 ± 9.0 days in LCM and 9.80 ± 8.04 days in LEV arm. In terms of baseline seizure frequency, types of seizure, sensory symptoms, automatism, history of stroke, head injury, imaging, and EEG abnormality, no significant difference was noted between both the groups. Structural epilepsy was found in 21 of the 35 LCM PWE and 22 of the 35 LEV PWE. The average daily dose of LCM was 385.71 ± 49.36 mg/day, and that of LEV was 1885.71 ± 501.25 mg/day [Table 1].
| Variables | Lacosamide n=35 (%) |
Levetiracetam n=35 (%) |
P-value |
|---|---|---|---|
| Age in years (Mean±SD) range | 38.20±16.62 (16–65) | 38.91±17.13 (16–65) | 0.86 |
| Gender M: F ratio (%) | 24 (68.7): 11 (31.3) | 27 (77.1):8 (22.9) | 0.23 |
| Baseline seizure frequency (per month) | 3.37±1.84 | 3.54±3.04 | 0.77 |
| Type of seizure | |||
| FAS | 2 (5.7) | 2 (5.7%) | |
| FIAS | 5 (14.2) | 7 (20) | 0.6 |
| FBTCS | 28 (80) | 26 (74.3) | |
| Sensory symptoms (present/absent) | 6 (17.1):29 (82.9) | 2 (5.7):33 (94.3) | 0.13 |
| Automatism (present/absent) | 4 (11.4):31 (88.5) | 4 (11.4):31 (88.5) | 1.00 |
| History of stroke (present/absent) | 9 (25.8):26 (74.2) | 5 (14.3):30 (85.7 | 0.18 |
| History of head injury (present/absent) | 3 (8.6):32 (91.4) | 3 (8.6):32 (91.4) | 1.00 |
| Daily doses, mg (Mean±SD) | 385.71±49.36 | 1885.71±501.25 | NA |
| EEG abnormality (present/absent) | 8 (22.9):27 (77.1) | 8 (22.9):27 (77.1) | 0.61 |
| CT/MRI brain abnormality (present/absent) | 21 (60):14 (40) | 22 (62.9):13 (37.1) | 0.50 |
FAS: Focal aware seizure, FIAS: Focal impaired awareness seizure, FBTCS: Focal to bilateral seizure tonic clonic seizure, CT: Computed tomography, MRI: Magnetic resonance imaging, EEG: Electroencephalographic, SD: Standard deviation, NA: Not applicable
Underlying comorbidities such as diabetes mellitus, hypertension, tuberculosis, cardiac disease, chronic kidney disease, and various biochemical parameters were non-significant between the groups.
Imaging and EEG finding
All 70 participants underwent neuroimaging using both CT scan and MRI. MRI was acquired for 61 PWE (87.1%), and CT scan for the rest 9 PWE. Forty-three (61.4%) PWE had a positive finding in neuroimaging. Of these, a potentially epileptogenic lesion was detected in 39 (56%) and a nonepileptogenic abnormality in 4 (5.4%) PWE.
Routine interictal vEEG was performed in all 70 PWE. The initial EEG recorded was abnormal in 16 PWE (22.8%); 8 (11.4%) in LCM group and 8 (11.4%) in LEV group. Specific EEG abnormality were slowing (generalized as well as focal) recorded in 9 (12.9%), focal epileptiform discharges in 3 (4.3%), multifocal epileptiform discharges in 2 (2.9%), and PLEDs in 2 (2.9%) PWE.
Primary outcome variable (efficacy)
Both primary and secondary outcome measures were calculated for 66 PWE. Mean seizure frequency in the LCM group decreased from 3.33 ± 1.88 to 0.24 ± 0.56 (P = 0.001) at 1 month, 0.48 ± 0.93 (P = 0.001) at 3 months, and 0.85 ± 1.09 (P = 0.001) at 6 months. Similarly, seizure frequency reduced in the LEV group from 3.61 ± 3.12 to 0.21 ± 0.60 (P = 0.001) at 1 month, 0.58 ± 1.2 (P = 0.001) at 3 months, and 0.94 ± 1.24 (P < 0.001) at 6-month follow-up. On comparison of seizure reduction between two studied ASM; we found no significant difference between LCM and LEV arm at baseline (P = 0.77, 95% CI – 0.9628–1.5228), at 1 month (P = 0.83, 95% CI - 0.301–0.25), 3 month (P = 0.73, 95% CI – 0.4180–0.6180), and 6 month (P = 0.74, 95% CI – 0.4733–0.6533) follow-up. Hence, in view of the non-inferiority margin of −0.5, we concluded that seizure reduction in both the groups was similar and LCM group was found non-inferior than LEV group [Table 2 and Figure 2a].
| OP | LCM (n=33) |
P-value compared with baseline | LEV (n=33) |
P-value compared with baseline | P-value between groups |
|---|---|---|---|---|---|
| Baseline (Seizure frequency per month) | 3.33±1.88 | 3.61±3.12 | 0.77 | ||
| OP 1 (1 month) | 0.24±0.56 | <0.001 | 0.21±0.60 | <0.001 | 0.83 |
| OP 2 (3 months) | 0.48±0.93 | <0.001 | 0.58±1.2 | <0.001 | 0.73 |
| OP 3 (6 months) | 0.85±1.09 | <0.001 | 0.94±1.24 | <0.001 | 0.74 |
LCM: Lacosamide, LEV: Levetiracetam, OP: Observation period
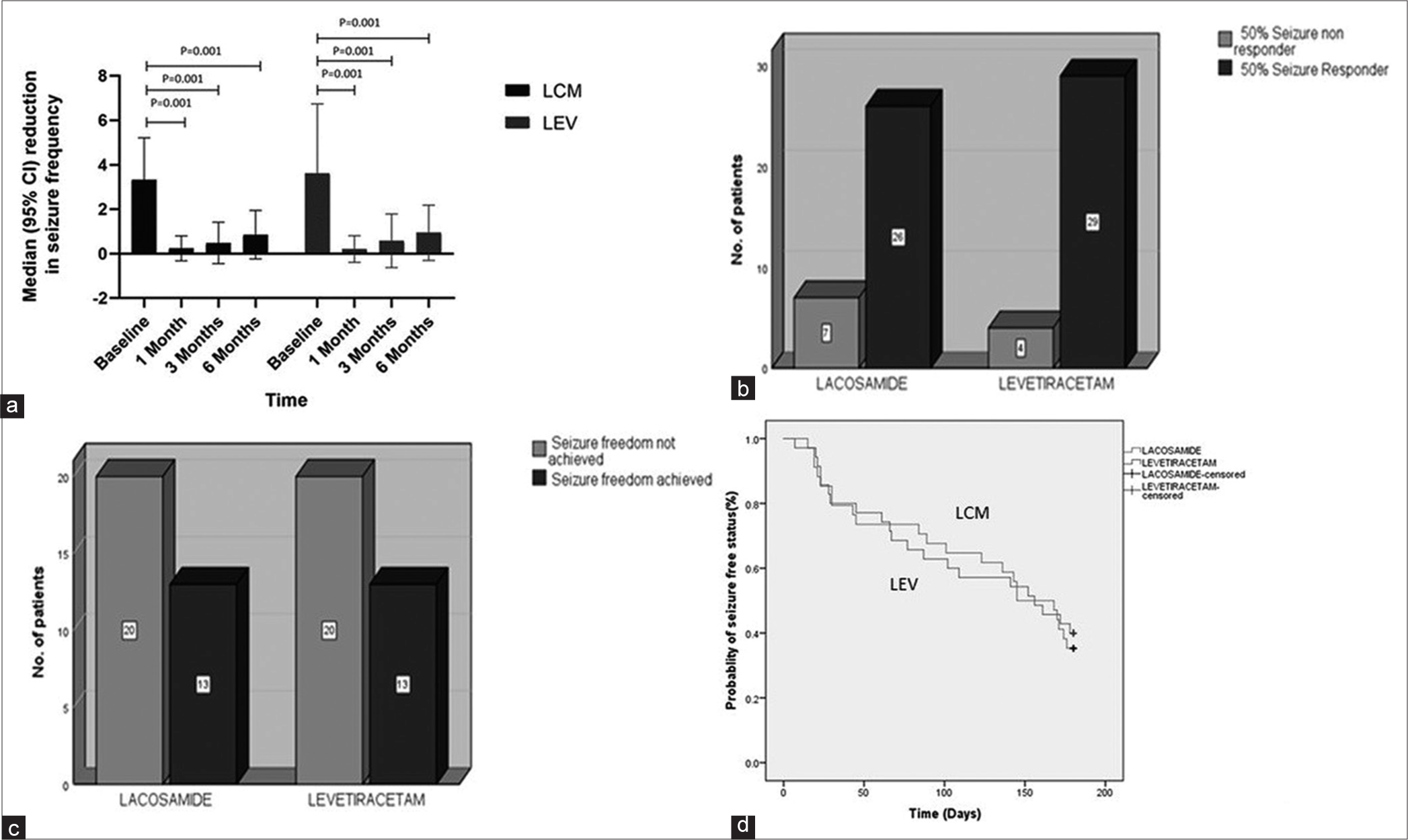
- (a) Error bar diagram showing significant median seizure frequency reduction from baseline to 6-month follow-up in LCM and LEV group, (b) 50% and (c) 100% of responder rates during the treatment and maintenance periods among PWE taking LCM and among those taking LEV showing non-significant difference between both the groups, and (d) Kaplan–Meier survival estimates for time to first focal seizure and comparison between LCM and LEV. PWE: People with epilepsy, LCM: Lacosamide, LEV: Levetiracetam.
Secondary outcome variable (seizure free status)
In the LCM arm, 26(78.9%) and in the LEV arm, 29(87.9%) out of 33 PWE achieved 50% seizure reduction from baseline to 6 months (P = 0.51), which was non-significant. Seizure freedom at 6 months was achieved in 13 (43.3%) of 33 PWE in both LCM and LEV arms non-significant difference (P = 1), which indicates LCM was non-inferior to LEV when compared for seizure freedom [Figure 2b and c].
Estimate of mean seizure-free days
The risk of developing focal seizure during the 180-day (6 months) treatment period was compared between LCM and LEV groups. The mean seizure-free days were 124.41 (95% CI: 102.91–145.90) and 121.28(95% CI: 99.80–142.77), respectively for LCM and LEV group (P = 0.82, HR = 0.93) [Figure 2d].
Secondary outcome variable treatment emergent adverse events (TEAEs)
During the treatment period, 10 (30.3%) of the 33 PWE receiving LCM reported a TEAEs compared with 15 (45.4%) of the 33 PWE receiving LEV. Majority of these were mild-to-moderate in severity. The most common LCM-related TEAEs were central nervous system or gastrointestinal related. No serious TEAEs or medication noncompliance were noted in any group [Table 3].
| AEs | LCM n= 10 (%) |
LEV n= 15 (%) |
|---|---|---|
| Serious AE | 0 | 0 |
| Fatigue | 3 (9.1) | 0 |
| Dyspepsia | 2 (6.1) | 0 |
| Headache | 2 (6.1) | 0 |
| Dizziness | 1 (3.03) | 0 |
| Pruritus | 1 (3.03) | 0 |
| Decrease sleep | 1 (3.03) | 0 |
| Somnolence | 0 | 8 (24.2) |
| Drowsiness | 0 | 5 (15.1) |
| Behavioral abnormality | 0 | 2 (6.1) |
TEAEs: Treatment-emergent adverse events, AEs: Adverse events, LCM: Lacosamide, LEV: Levetiracetam
DISCUSSION
This study suggests that LCM is non-inferior to LEV as monotherapy for new onset focal seizure in terms of seizure frequency reduction, achievement of seizure freedom, and drug tolerability. About 50% or more seizure reduction (78.9% in LCM vs. 87.9% in LEV, P = 0.51) and seizure freedom up to 6 months (43.3% in LCM vs. 43.30% in LEV, P = 1) was also equivalent in both groups. The risk of developing a focal seizure recurrence during the 180-day monotherapy as well as mean seizure-free days (P = 0.82) was also similar. Our study findings are in concurrence with the three major comparative studies; Baulac et al. comparing LCM monotherapy and controlled release carbamazepine (CBZ CR) in newly diagnosed epilepsy,[9] Ben-Menachem et al. comparing the same two drugs,[13] and Del Bianco et al. comparing LCM to LEV in elderly population of focal seizure.[3] The proportion of PWE in the full analysis (LCM arm [n = 444), vs. CBZ CR arm [n = 442]) set predicted seizure-free at 6 months by the Kaplan–Meier study as 90% in LCM arm and 91% in CBZ CR (absolute treatment-difference – 1.3%) in Baulac et al. study which was identical to our finding. Long-term (median 2 years) LCM monotherapy was efficacious and well-tolerated in individuals with newly diagnosed epilepsy, according to Ben-Menachem et al. (LCM arm [n = 211], CBZ CR arm [n = 180]). The percentage of PWE with 12- and 24-month seizure freedom from the first dose in Kaplan–Meier estimate were 50.8% and 47.0% on LCM and 54.9% and 50.9% on CBZ CR, respectively. Del Bianco et al. study compared 22 PWE of LCM monotherapy with 24 PWE of LEV monotherapy.
At 12 months of follow-up, mean monthly seizure frequency reduced from 4.23 ± 8.53 to 0.33 ± 0.9 (P = 0.001) in LCM group and from 2.29 ± 6.11 to 0.2 ± 0.81(P = 0.001) in LEV group with 72.7% LCM PWE and 70.8% in LEV group achieved seizure-freedom.
Unlike other ASMs, LCM selectively enhances sodium channel inactivation slowly that results in stabilization of hyperexcitable neuronal membranes and inhibition of neuronal firing without affecting physiological function.[14] LCM is rapidly absorbed with high oral bioavailability (100%) for a dose up to 800 mg irrespective of food intake with a consistent pharmacokinetic profile and predictable plasma concentration.[14] Severe TEAEs such as cardiotoxicity were not noted in any group. None of the PWE discontinued medication due to drug-related side effects. These safety findings matched those of a pooled analysis of safety data from the three randomized, double-blind, and placebo-controlled trials by Biton et al.[8] Dizziness was the most common symptom (30.6%), followed by nausea (11.4%), diplopia (10.5%), fatigue (7.2%), ataxia (7.2%), tremor (6.2%), and memory impairment (1.5%). In Brodie et al. study the common side effects in LCM group were headache (20.7%), fatigue (16.5%), somnolence (11.2%), and dizziness (10.9%) with a drug discontinuation rate <1%.[15]
Thus, it may be concluded that LCM is a good long-term monotherapy option for individuals with focal seizures of any age group with other comorbidities with minimal risk of drug interactions.[14]
Limitations of study
New onset seizure patients were included in our study and the calculated pre-treatment seizure frequency may not be the true scenario in all the cases, especially in cases with <1 month onset. Long-term follow-up assessment was not done in our study which was conducted in a few of the comparative trials discussed above.[3,4,11] However, based on the known tolerability of the drugs and good compliance in the study population, we can predict a similar finding even on long-term follow-up.
CONCLUSION
This is the first head-to-head clinical trial directly comparing LCM with LEV monotherapy in new-onset focal epilepsy in adult PWE irrespective of age. The unique properties of LCM make it an appropriate first line therapy rather than an adjuvant therapy in management of focal seizures. Large scale trials may be conducted for more robust evidence in this regard.
Declaration of patient consent
The authors certify that they have obtained all appropriate consent.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Lacosamide as adjunctive therapy for partial-onset seizures: A randomized controlled trial. Epilepsia. 2010;51:958-67.
- [CrossRef] [PubMed] [Google Scholar]
- Adjunctive lacosamide for partial-onset seizures: Efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50:443-53.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term efficacy and safety of lacosamide and levetiracetam monotherapy in elderly patients with focal epilepsy: A retrospective study. Epilepsy Behav. 2019;94:178-82.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48:1308-17.
- [CrossRef] [PubMed] [Google Scholar]
- Lacosamide in pediatric and adult patients: Comparison of efficacy and safety. Seizure. 2013;22:210-6.
- [CrossRef] [PubMed] [Google Scholar]
- Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54:551-63.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term safety and efficacy in patients with uncontrolled partial-onset seizures treated with adjunctive lacosamide: Results from a Phase III open-label extension trial. Epilepsia. 2012;53:521-8.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and tolerability of lacosamide as adjunctive therapy for adults with partial-onset seizures: Analysis of data pooled from three randomized, double-blind, placebo-controlled clinical trials. Epilepsy Behav. 2015;52:119-27.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled-release carbamazepine in patients with newly diagnosed epilepsy: A phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2017;16:43-54.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and tolerability of lacosamide in the concomitant treatment of 130 patients under 16 years of age with refractory epilepsy: A prospective, open-label, observational, multicenter study in Spain. Drugs R D. 2012;12:187-97.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term exposure and safety of lacosamide monotherapy for the treatment of partial-onset (focal) seizures: Results from a multicenter, open-label trial. Epilepsia. 2016;57:1625-33.
- [CrossRef] [PubMed] [Google Scholar]
- Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531-42.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term safety and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy. Epilepsia. 2019;60:2437-47.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical pharmacokinetic and pharmacodynamic profile of lacosamide. Clin Pharmacokinet. 2015;54:901-14.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsy. Neurology. 2007;68:402-8.
- [CrossRef] [PubMed] [Google Scholar]






