Translate this page into:
Mania associated with complicated hereditary spastic paraparesis
Address for correspondence: Dr. Raghavendra B Nayak, Department of Psychiatry, Jawaharlal Nehru Medical College, KLE University, Belgaum - 590 010, Karnataka, India. E-mail: rbn.psych@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hereditary spastic paraparesis (HSP) is an inherited group of neurological disorders with progressive lower limb spasticity. HSP can be clinically grouped into pure and complicated forms. Pure HSP is one without any associated neurological/psychiatric comorbidity. Depression is the most common psychiatric comorbidity. Presence of mania or bipolar affective illness with HSP is a rare phenomenon. We report a case of a 17-year-old boy who presented with classical features of HSP with complaints of excessive happiness, irritability, increased self-esteem and decreased sleep since 1 month. The patient also had complex partial seizure ever since he had features of HSP. The patient's father and younger sister suffer from pure HSP. The patient was diagnosed to have first episode mania with complicated HSP. The details of treatment and possible neurobiology are discussed in this case report.
Keywords
Complicated hereditary spastic paraparesis
hereditary spastic paraparesis
mania
Introduction
Hereditary spastic paraparesis (HSP) or Strümpell–Lorrain syndrome is an inherited group of neurological disorders with progressive lower limb spasticity. HSP is classified into pure/uncomplicated and complicated/complex forms. HSP is transmitted through an autosomal-dominant (AD), autosomal-recessive (AR) or X-linked (XL) manner. An AD transmission accounts for 70–80% of all HSP.[1] It affects all the age groups, and the prevalence of HSP ranges from 2.0 to 9.6/100,000 depending on different diagnostic criteria used and populations studied.[2] The cardinal abnormalities of patients with HSP include spasticity, hyperreflexia, urinary bladder disturbance (hypertonic bladder) and extensor plantar responses with weakness of a pyramidal distribution in the lower limbs. We report a case of a 17-year-old boy who presented with HSP and mania. The possible association of HSP with bipolar disorder has not been frequently addressed in the medical literature.
Case Report
A 17-year-old boy reported to the Department of Psychiatry with complaints of excessive happiness, irritability, increased self-esteem and decreased sleep since 1 month. The patient was known case of HSP with epilepsy since 7 years. He was born of a nonconsanguinous marriage. Details of family are mentioned in the family genogram [Figure 1]. The patient had episodes of staring, twitching around the mouth, grimacing followed by loss of consciousness and fall followed by involuntary jerky movements of the extremities. His seizures and neurological illness had both started simultaneously. The patient was taking Tab carbamazepine 400 mg/day for the last 5 years. Seizure episodes decreased after starting carbamazepine from three to four episodes in a year to total absence of episodes in last 3 years. The patient's father and sister also have HSP without any diagnosable psychiatric or epileptic disorders. All the affected family members had onset of HSP around 12 years of age, which had progressed for 3–4 years and, later, the condition became static. The patient's mother had a history of paranoid schizophrenia; she had committed suicide 8 years ago.
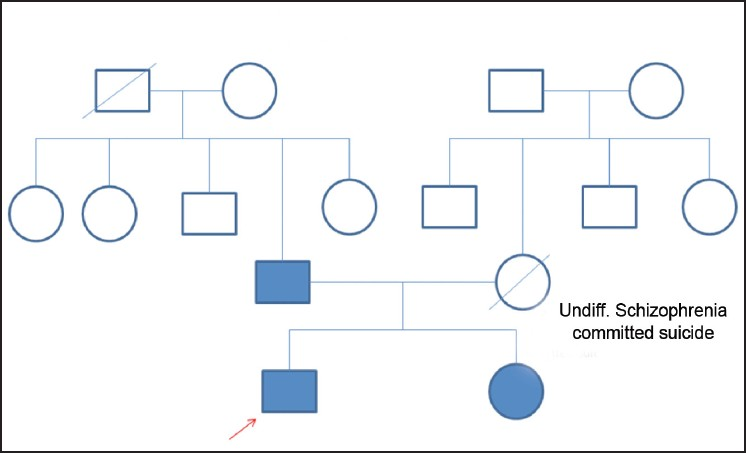
- Family genogram
On general physical examination, the patient was moderately built and nourished, with normal vital parameters. He was conscious and well-oriented during examination. His neurological examination revealed that both his limbs were equally affected. There was increased muscle tone in the hamstring group and in the ankle region, positive patellar clonus and Babinski's sign, brisk deep tendon reflexes, wasting of distal muscle groups and spastic gait. There were no cranial nerve deficits, cerebellar signs and signs of meningeal irritation. On mental status examination, he was well kempt, had increased psychomotor activity, pressure of speech, irritability, increased self-esteem and delusion of grandiosity. When baseline Young's Mania Rating Scale (YMRS) was applied, the score was 26, and his baseline hematological and biochemical investigations were within normal limits. Computed tomography imaging did not reveal any abnormalities. Diagnosis of the first episode of mania with psychotic symptoms was made using ICD-10 DCR criteria.[3] The patient was independently interviewed by two competent psychiatrists to rule out any other psychiatric disorders, but screening instruments like SCID or MINI were not used. The patient was given Tab Sodium Valproate 500 mg bid/day and Tab Olanzapine 15 mg/day. Valproate was cross-tapered with carbamazepine over a period of 2 weeks. Considering the poor response during the first 2 weeks with Olanzapine and a similar observation by the family members, the treating team decided to change Olanzapine to Tab Trifluoperazine 10 mg bid/day and Tab Trihexyphenidyl 2 mg bid/day. The patient showed good improvement to the change of these medications and his YMRS score decreased to 10 after 10 days. Then, the patient was discharged and was asked to come for a follow-up. Antipsychotic medications were stopped after 3 months and only Valproate was continued. The patient is regularly followed-up for 6 months and his symptoms have shown remission. There are no recurrences of seizure episodes.
Discussion
On evaluation, the patient had severe mania with a YMRS score of 26. The presence of severe manic symptoms warranted the use of a mood stabilizer Valproate in the first episode itself.[4] Tab Valproate was cross-tapered along with tab carbamazepine because, clinically, we use Valproate more commonly as compared with carbamazepine. The risk of changing carbamazepine was discussed with the family members as carbamazepine was the better choice for complex partial seizures.[5] Ideally, in our case, Olanzapine should have been continued for 4-6 weeks as per the guidelines, but due to inadequate response and request by the family members, the treating team decided to change Olanzapine to Trifluoperazine.
In 1981, Harding et al.[6] detailed the clinical evaluation of 22 families and suggested criteria for classifying HSP into pure and complicated forms. Pure HSP is without any associated neurological/psychiatric comorbidity. Complicated hereditary spastic paraparesis has been associated with many conditions, including optic atrophy, retinopathy, extrapyramidal disease, amyotrophy, dementia, ataxia, mental retardation, deafness, icthyosis, peripheral neuropathy, epilepsy and psychiatric disorders.[2] The major neuropathological feature of pure HSP is axonal degeneration, which is maximal in the terminal portions of the longest descending (corticospinal tracts) and ascending (dorsal column pathways) tracts within the spinal cord. The genetic profile of HSP reveals 32 HSP loci and 11 disease-related genes. Of the loci, 13 are responsible for AD HSP and 4 for XL-HSP. A group of the proteins they encode localize to the endosomal membrane traffic compartment, suggesting that the long axons of the corticospinal tract may be especially vulnerable to endosomal dysfunction and lead to physical symptoms.[7]
However, psychiatric manifestations are rare but mood disorders, organic personality changes, dementia, psychosis and mental retardation may be associated with this condition. Depression is the most common psychiatric comorbidity. A study of 48 patients with HSP revealed that 58% of the patients suffer from depression. Most patients had mild to moderate depression, and only one had a severe depressive episode.[8] Bipolar affective disorder is a rare association with HSP. There are only two reported cases in the literature.[910] A study in the Irish population reports that patients with HSP are at a higher risk of developing psychosis.[11]
The neurobiology of HSP with psychiatric manifestations is not clear. Psychosis is common in families with Kjellin's syndrome and SPG4-HSP.[11] In the above Irish study, patients had ventricular enlargement and mild generalized atrophy on cranial imaging.[11] A clinicopathological study of a family in Japan showed that all the patients had hypoperfusion in the frontal lobes and thalami on SPECT, and neuropathologic findings revealed thin corpus callosum and degeneration in the thalamic dorsomedial nuclei as well as degeneration of the corticospinal tracts.[12] Reports suggest that white matter abnormalities in the brain are commonly noted in patients with bipolar affective disorder. The tracts connecting the frontal lobe and subcortical structures are implicated in the neurobiology of bipolar affective disorder.[13] White matter abnormalities could be one common factor between HSP and mania.
Sometimes, mania and epilepsy together can occur and are believed to be having a common underlying neurobiology, like kindling paradigm and changes in second-messenger systems.[14] But, the prevalence of true manic depressive illness in epilepsy has been demonstrated to be in line with that reported in the general population (about 2%) and, therefore, is difficult to consider the common underlying neurobiology.[15] The patient's mother had paranoid schizophrenia, which could be another factor for the above association, because affective disorders are reported in the same family who have schizophrenia spectrum disorders, but this is uncommon.
Conclusions
Mania is a coincidental occurrence that needs evaluation for association with HSP. However, there may be common underlying factors as suggested by this case. Thus, the diagnosed cases of HSP need detailed evaluation for psychiatric morbidity.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- The prevalence of ‘pure’ autosomal dominant hereditary spastic paraparesis in the island of Ireland. J Neurol Neurosurg Psychiatry. 2002;72:43-6.
- [Google Scholar]
- Hereditary spastic paraparesis: a review of new developments. J Neurol Neurosurg Psychiatry. 2000;69:150-60.
- [Google Scholar]
- The ICD-10 Classification of Mental and Behavioural Disorders Diagnostic criteria for research. 1993. Geneva: World Health Organization; Available from: http://www.who.int/classifications/icd/en/GRNBOOK.pdf
- [Google Scholar]
- American Psychiatric Association. 2002. Practice guideline for the treatment of patients with bipolar disorder. (2nd ed.). Available from: http://www.psychiatryonline.com/pracGuide/loadGuidelinePdf
- [Google Scholar]
- A Comparison of Valproate with Carbamazepine for the Treatment of Complex Partial Seizures and Secondarily Generalized Tonic–Clonic Seizures in Adults. N Engl J Med. 1992;327:765-71.
- [Google Scholar]
- Hereditary pure spastic paraplegia: A clinical and genetic study of 22 families. J Neurol Neurosurg Psychiatry. 1981;44:871-83.
- [Google Scholar]
- The hereditary spastic paraplegia proteins NIPA1, spastin and spartin are inhibitors of mammalian BMP signalling. Human Molecular Genetics. 2009;18:3805-21.
- [Google Scholar]
- The prevalence of depression in hereditary spastic paraplegia. Clin Rehabil. 2009;23:857-61.
- [Google Scholar]
- Hypomanic behaviour associated with familial spastic paraplegia. Eur Arch Psychiatry Neurol Sci. 1988;238:28-30.
- [Google Scholar]
- Hereditary spastic paraplegia, bipolar affective disorder and intellectual disability: A case report. J Intellect Disabil. 2008;12:41-8.
- [Google Scholar]
- Hereditary spastic paraplegia with frontal lobe dysfunction: A clinicopathologic study. Neurology. 2004;63:2149-52.
- [Google Scholar]
- A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neurosci Biobehav Rev. 2010;34:533-54.
- [Google Scholar]
- Bipolar Disorder and Epilepsy: A Bidirectional Relation.Neurobiological Underpinnings, Current Hypotheses, and Future Research Directions? Neuroscientist. 2007;13:392-404.
- [Google Scholar]
- Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord. 2000;59:S5-30.
- [Google Scholar]






