Translate this page into:
Clinicoradiological profile and outcome of cavernous sinus syndrome with coronavirus disease-2019-associated rhino-orbito-cerebral mucormycosis
*Corresponding author: Sulena Sulena, Department of Neurology, Guru Gobind Singh Medical College, Faridkot, Punjab, India. sulenasingh@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Sulena S, Dhawan M, Singh N, Arora H, Singh G, Aggarwal V, et al. Clinicoradiological profile and outcome of cavernous sinus syndrome with coronavirus disease-2019-associated rhino-orbito-cerebral mucormycosis. J Neurosci Rural Pract 2022;13:730-9.
Abstract
Objective:
With coronavirus disease 2019 (COVID-19) pandemic across the world, there had been an exponential increase in rhino-orbito-cerebral mucormycosis (ROCM). Extension of infection to cavernous sinus leads to cavernous sinus syndrome (CSS). This study aims to describe incidence, clinicoradiological profile, and outcome of CSS positive along with comparative analysis of CSS negative COVID-19-associated ROCM.
Material and Method:
This was a prospective and observational study conducted from May 1, 2021, to July 31, 2021. Subjects included ROCM with active or recovered COVID-19 (past 6 weeks) and were categorized and staged. CSS was defined as involvement of two or more of third, fourth, fifth, or sixth cranial nerve with one each direct and indirect qualitative neuroradiological features. Clinicoradiological features of CSS-positive and negative COVID-19-associated ROCM groups were compared.
Results:
Incidence of CSS with COVID-19-associated ROCM was 28%. Mean age of subjects was 44 ± 15 years with 60% being males and 73% were proven ROCM. Significant differences seen across the CSS-positive and negative groups were ocular, nasal, and cerebral findings including eyelid and periocular discoloration, ptosis, proptosis, ophthalmoplegia, nasal discharge, mucosal inflammation, and fever. Oculomotor, trochlear, and abducens nerves were significantly involved more in CSS-positive group. Significant radiological findings across two groups included indirect features in orbit, nose, and paranasal sinuses along with direct features in cavernous sinus. Surgical intervention was more common in CSS-positive group. Mortality in CSS-positive group at 8–24 weeks was 13 and 27%, respectively.
Conclusion:
Extension of ROCM to CSS was more common in young males in advanced stages of proven ROCM with concurrent COVID-19. CSS-positive group had significant difference in clinicoradiological features involving orbit, nose, paranasal sinuses, and central nervous system as compared to CSS-negative group. This study highlights the need to develop an objective scoring system considering clinical and radiological features for diagnosis of CSS with COVID-19-associated ROCM.
Keywords
Cavernous sinus syndrome
COVID-19-associated ROCM
COVID-19
Rhino-orbito-cerebral Mucormycosis
Cavernous sinus
Neuroinfections
INTRODUCTION
During the pandemic of coronavirus disease 2019 (COVID-19) across the world since 2019, there is an exponential increase in the opportunist fungal infection-rhino-orbito-cerebral mucormycosis (ROCM).[1-3] This is an acute angioinvasive infection caused by fungal order Mucorales in patients with a background of immunocompromised conditions such as uncontrolled diabetes mellitus, use of corticosteroids and immunosuppressive drugs, immunodeficiency states, hematological malignancies, solid organ malignancies, or transplantation.[1] Concurrent COVID-19 provides a metabolically suitable environment for the growth of inhaled sporangiophores to germinate into hyphae and spread through tissue invasion, thrombosis, and necrosis to adjoining areas.[4]
The spectrum of ROCM ranges from limited nasal and paranasal sinuses or rhino-orbital disease to widespread rhino-orbito-cerebral disease central Nervous system (CNS involvement).[5,6] In India, the prevalence of mucormycosis (0.14 cases/1000 population) has been more than the developed nations and contributed to 80% of COVID-19-associated ROCM during second peak of pandemic.[1] The extension of infection to brain increases mortality rate of ROCM to 60%, but with early and aggressive surgical and medical treatment, it can be reduced to 30%.[7,8] CNS involvement occurs in 21–37% of ROCM by various routes of spread of infection including sinuses, blood vessels, nerves, cribriform plate of ethmoid bone, or pterygopalatine fossa.[9,10]
Involvement of cavernous sinus by ROCM leads to cavernous sinus syndrome (CSS). CSS is a group of signs and symptoms involving variable combinations of cranial nerves 3, 4, 5, and 6 along with oculosympathetic and parasympathetic plexii in cavernous sinus.[11] Cavernous sinus is a dangerous and difficult site for treatment and surgery due to its complex neurovascular structures. There are various causes of CSS including vascular diseases, neoplasms, infections, and inflammatory diseases. In Indian scenario, infections especially fungal constitutes an important etiology for CSS.[12] There is insufficient data on clinicoradiological description of involvement of cavernous sinus in COVID-19-associated ROCM. To the best of our knowledge, there had not been any prospective study on CSS in ROCM and present day scenario urges for one to understand the clinical progression of the disease.
This study aims to describe incidence and clinicoradiological profile and outcome of CSS-positive along with comparative analysis of CSS-negative COVID-19-associated ROCM. The early recognition of clinicoradiological features suggestive of CSS in COVID-19-associated ROCM may affect the course of an otherwise potentially fatal disease.
MATERIALS AND METHODS
This prospective and observational study was conducted in the division of neurology in a tertiary level hospital from resource limited rural belt of North India. This 600 bedded hospital is a dedicated Level III center for the management of COVID-19.
The study was conducted from May 1, 2021, to July 31, 2021, during the second peak of COVID-19 pandemic. The study sampled consecutive active (positive SARS-CoV-2 on reverse-transcription polymerase chain reaction analysis in the nasopharyngeal or oropharyngeal swabs) or recovered COVID-19 in the past 6 weeks with features suggestive of ROCM [Supplementary file-Annexure 1]. The study excluded subjects who were more than 6-week post-COVID-19 infection and had any other cause of CSS such as neoplasm, inflammation, or bacterial infections to avoid the selection bias.
ROCM was diagnosed and categorized as: [13]
Possible cases: Clinical features suggestive of ROCM with background of concurrent or recently (<6 weeks) diagnosed case of COVID-19.
Probable cases: Features in possible category and positive findings on diagnostic nasal endoscopy or neuroimaging.
Proven cases: Features in probable category and microbiological confirmation of mucormycosis on direct microscopy, culture growth, or histopathological examination with special stains.
Staging of ROCM was done as proposed in a recent editorial by Honavar et al.[13]
ROCM was divided into CSS-positive and negative group depending on the involvement of cavernous sinus. CSS was defined as group of signs and symptoms suggestive of involvement of two or more of third, fourth, fifth (V1 and V2), or sixth cranial nerve with each one direct and indirect qualitative neuroradiological features [Supplementary file-Annexure 2]. The proposed neuroradiological features were prepared after a thorough literature review.[14-16] CSS was classified using Ishikawa classification [Supplementary file-Annexure 3].[12,14]
Clinical examination, procedures, and investigations were done using recommended government guidelines for COVID-19. Verbal and written informed consent was taken from subjects or her/his guardians. Clinicodemographic profile included age, gender, residential address, occupation, and presenting complaints. Ophthalmological, neurological (including higher mental functions, cranial nerves, motor, sensory, and extrapyramidal system), and ear, nose, and throat examination was performed. Vision loss was defined as visual acuity lower than finger counting at less than a half meter. The laboratory investigations included blood cell counts, hepatic and renal function tests, fasting lipid profile, and chest x-ray. Diagnostic nasal endoscopy was done for tissue diagnosis.
Neuroimaging included either computerized tomography (CT scan) head and orbit (Siemens Somatom Perspective 128 slice) or magnetic resonance imaging of brain (MRI brain) (Siemens Magnetom Avanto 1.5 Tesla). Magnetic resonance venogram was done in patient with high index of suspicion of venous thrombosis and clinically stable to be shifted to the radiology department. The contrast-enhanced neuroimaging was done only in subjects with normal renal function tests. The imaging studies were assessed for features suggestive of ROCM and CSS independently by a trained radiologist with an experience of 8 years, and a final diagnosis was reached after discussion and consensus with multidisciplinary team consisting of neurologist, ophthalmologist, otorhinolaryngologist, and neurosurgeon.
The short-term outcome was assessed at 8 weeks as good, if discharged from the hospital with clinicoradiological improvement and no further progression of disease. Poor outcome included expired, referred, or left against medical advice for any reasons. The long-term outcome for mortality was assessed with a telephonic follow-up at 24 weeks.
This study aims to describe incidence and clinicoradiological profile and outcome of CSS along with comparative analysis of CSS-negative COVID-19 associated ROCM. The null hypothesis assumed no difference for sociodemographic and clinicoradiological profile variables across these patient groups.
The patient data pertaining to sociodemographic and other clinical variables were entered in the form of a data matrix in Microsoft® Excel® and analyzed using IBM® SPSS® v 20.0.0. The descriptive statistics were presented as frequencies and percentages/means and standard deviations as appropriate. Chi-square/Fisher’s exact test was used to assess association among categorical variables and Independent Samples T test/ Mann–Whitney U test for exploring difference of continuous variables across two groups. A two-sided P < 0.05 was considered as statistically significant.
RESULTS
There were a total 2190 of COVID-19-associated admissions during the study period.
After screening, 54 subjects with COVID-19-associated ROCM were selected [Figure 1]. Incidence of CSS in COVID-19-associated ROCM was 28% (bilateral CSS in 27%) with whole cavernous sinus being most common site. Incidence of ROCM in COVID-19 was 2.4%.
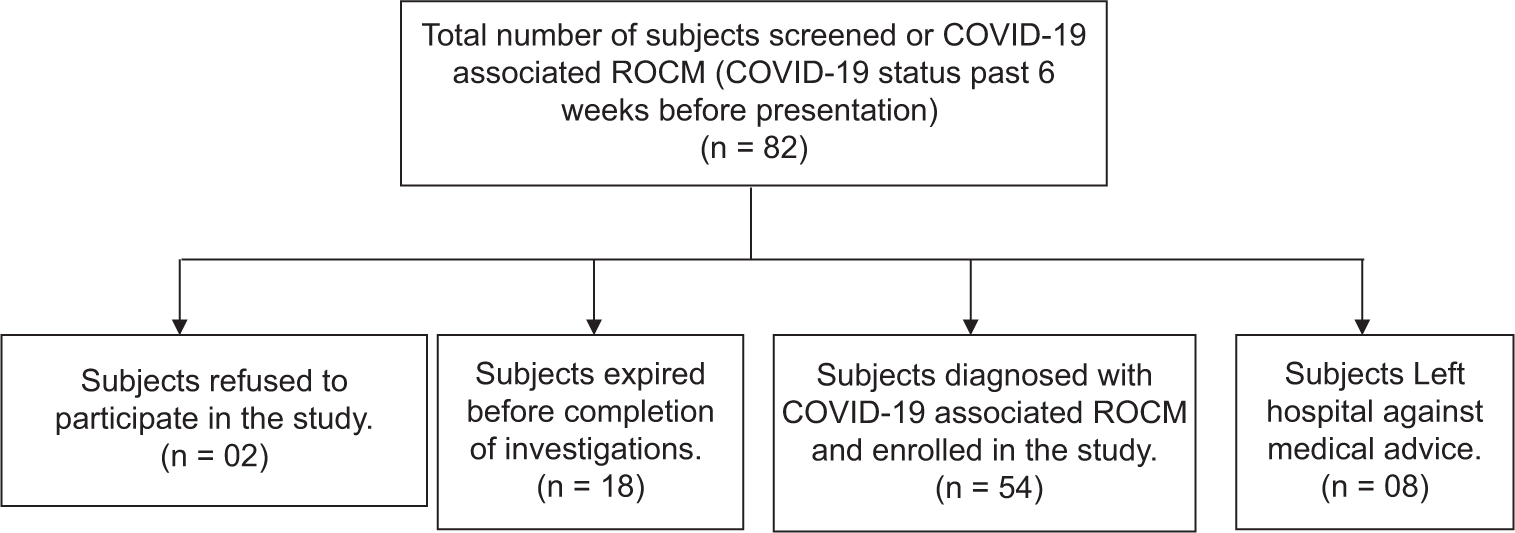
- Flowchart showing screening and enrollment of subjects with COVID-19-associated rhino-orbito-cerebral mucormycosis.
[Table 1] shows clinicoepidemiological and risk factors profile of CSS-positive and negative groups. A statistically significant number of CSS positive group were in advanced stages of ROCM as compared to CSS-negative group (P < 0.005). Mean age in CSS-positive group (44 ± 15 years) was significantly lower than CSS-negative subjects (52 ± 11 years). Duration of symptoms onset (Median=10 days) was significantly longer in CSS-positive group. Only 1 subject (2%) had received first dose of COVID-19 vaccination and none had received two doses.
| S. no. | Parameter | CSS positive (n=15 subjects) | CSS negative (n=39 subjects) | P value |
|---|---|---|---|---|
| Number (%) | Number (%) | |||
| 1 | Age (years) | 44±15 | 52±11 | 0.028 |
| 2 | Gender (Males) | 9 (60) | 26 (67) | 0.646 |
| 3 | Covid Status | |||
| Positive | 12 (80) | 22 (56) | 0.108 | |
| Recovered/Suspect | 03 (20) | 17 (44 ) | 0.108 | |
| 4 | ROCM Category | 0.659 | ||
| Possible Mucormycosis | 0 (0) | 1 (3) | ||
| Probable Mucormycosis | 04 (27) | 15 (38) | ||
| Proven Mucormycosis | 11 (73) | 23 (59) | ||
| 5 | Stage of ROCM | |||
| Stage 1 | 0 (0) | 1 (2.5 ) | <0.005 | |
| Stage 2 | 0 (0) | 19 (49 ) | ||
| Stage 3 | 02 (13) | 17 (44 ) | ||
| Stage 4 | 13 (87) | 2 (5) | ||
| 6 | Risk Factors and Co morbidities | |||
| Diabetes Mellitus | 13 (87) | 37 (95 ) | 0.302 | |
| Use of Corticosteroid | 07 (47) | 11 (28) | 0.197 | |
| Mehanical Ventilation or Supplemental Oxygen | 01 (7) | 6 (15) | 0.393 | |
| Primary or Secondary Immunodeficiency | 01 (7) | 8 (21) | 0.221 | |
| Chronic Kidney Disease | 01 (7) | 2 (5) | 0.825 | |
| Acute Kidney Injury/Tuberculosis/Coronary Artery disease. | 00 (0)/00 (0)/00 (0) | 2 (5)/2 (5)/2 (5) | 0.371 | |
| Past Stroke/hypertension | 00 (0)/00 (0) | 3 (8)/3 (8) | 0.269 | |
| Transplantation History | 00 (0) | 1 (3 ) | 0.531 | |
| Hepatitis B | 01 (7) | 0 (0 ) | 0.104 | |
| Hepatitis C | 00 (0) | 4 (10) | 0.197 | |
| 7 | Duration of symptom onset (Mean±2S.D) days | 12±7 (Median=10 days) | 6±9 (Median=6 days ) | 0.005 |
| 8 | KOH Smear positive | 6 (40) | 15 (38) | 0.972 |
| 9 | Biopsy positive | 8 (53) | 15 (38) | 0.322 |
| 10 | Culture positive | 1 (7) | 0 (0 ) | 0.104 |
| 11 | Antifungals used | |||
| Posaconazole | 15 (100) | 33 (85 ) | 0.273 | |
| Liposomal Amphotericin B | 11 (73) | 15 (38) | 0.022 | |
| 12 | Surgical Treatment | 9 (60) | 3 (8) | 0.001 |
| 13 | Outcome | 0.322 | ||
| Good: Discharge | 8 (53) | 15 (38) | ||
| Poor: Death/Referred/Lama | 2 (13)/3 (20)/(13) | 7 (18)/3 (8)/14 (36 ) |
Clinical features of ocular and nasal findings including eyelid, periocular, and facial discoloration (n = 15.100%), ptosis (n = 12.80%), ophthalmoplegia (n = 12.80%), proptosis (n = 9.60%), and nasal discharge and mucosal inflammation (n = 5.33%) were significantly more common in CSS-positive as compared to CSS-negative group. Oculomotor (n = 13.87%), trochlear (n = 10.67%), and abducens nerve (n = 12.80%) were involved significantly more in CSS-positive group [Table 2]. Dilated fundus examination could be done in only ten subjects (18%) due to severe chemosis, proptosis, and hazy status of cornea in majority of these subjects. Fundus examination was normal in 70% and optic atrophy was seen in one subject each in CSS-positive and negative group.
| Clinical features | CSS positive (n=15 subjects) number (%) | CSS negative (n=39 subjects) number (%) | P value |
|---|---|---|---|
| Nasal Clinical features | 13 (87) | 32 (82) | 1.000 |
| Nasal Stuffiness | 6 (40) | 12 (31 ) | 0.519 |
| Foul Smell | 1 (7) | 0 (0) | 0.104 |
| Epistaxis | 0 (0) | 2 (5) | 0.371 |
| Nasal Discharge | 5 (33) | 3 (8) | 0.03 |
| Nasal Mucosal Erythema, Inflammation, Purple or Blue Discoloration, White Ulcer, Ischemia, or Eschar | 5 (33) | 3 (8) | 0.03 |
| Regional Pain-Orbit, Paranasal Sinus or Dental Pain | 11 (73) | 26 (67) | 0.637 |
| Ocular Clinical Features | 15 (100) | 32 (82) | 0.171 |
| Proptosis | 9 (60) | 7 (18) | 0.006 |
| Loss of vision | 11 (73) | 19 (49) | 0.103 |
| Ophthalmoplegia | 12 (80) | 14 (28) | 0.004 |
| Ptosis | 12 (80) | 9 (23) | 0.000 |
| Eyelid Periocular or Facial Edema | 15 (100) | 30 (77) | 0.125 |
| Eyelid, Periocular, Facial Discoloration | 6 (40) | 9 (23 ) | 0.02 |
| Systemic and Cerebral Features | 15 (100) | 38 (97) | 1.000 |
| Fever | 15 (100) | 30 (77 ) | 0.049 |
| Headache | 8 (53) | 15 (38) | 0.322 |
| Facial Pain | 8 (53) | 22 (56) | 0.839 |
| Facial Paresthesias, Anesthesia | 9 (60) | 15 (38 ) | 0.154 |
| Facial Palsy | 1 (7) | 2 ( 5) | 0.825 |
| Altered Sensorium, Paralysis, Focal Seizures | 5 (33) | 7 ( 18) | 0.223 |
| Cranial nerve | CSS positive subjects | CSS negative subjects | P value | ||
|---|---|---|---|---|---|
| Unilateral number (%) | Bilateral number (%) | Unilateral number (%) | Bilateral number (%) | ||
| Cranial nerve 2 | 9 (60) | 2 (13) | 17 (44) | 2 (5) | 0.103 |
| Cranial nerve 3 | 9 (60) | 4 (27) | 11 (28) | 2 (5) | 0.000 |
| Cranial nerve 4 | 8 (53) | 2 (13) | 9 (23) | 1 (3) | 0.005 |
| Cranial nerve 5 | 8 (53) | 1 (7) | 12 (31) | 4 (10) | 0.210 |
| Cranial nerve 6 | 8 (53) | 4 (27) | 17 (43) | 1 (3) | 0.025 |
| Cranial nerve 7 | 1 (7) | 0 | 2 (5 ) | 1 (3) | 0.897 |
| Cranial nerve 9-12 | 1 (7) | 0 | 2 (5 ) | 1 (3) | 0.897 |
MRI brain, MRI brain with gadolinium contrast, and both CT and MRI brain were performed more commonly in CSS-positive group. Significant indirect qualitative radiological findings in the orbit were fat and muscle infiltration, enhanced soft-tissue mass in orbital apex, proptosis, preseptal cellulitis, enhancement of orbital muscles, and optic neuritis [Table 3].
| Neuroimaging | CSS positive (n=15 subjects) | CSS negative (n=39 subjects) | P value |
|---|---|---|---|
| Imaging | Number (%) | Number (%) | |
| CT head and Orbit | 10 (67) | 29 (74) | 0.736 |
| MRI Brain and Orbit | 12 (80) | 18 (46) | 0.025 |
| Both CT & MRI brain | 7 (47) | 7 (18) | 0.043 |
| MRI Brain with Contrast | 9 (60) | 11 (28) | 0.031 |
| MR Venogram | 3 (20) | 0 (0) | 0.005 |
| 3B. Neuroimaging Features | |||
| 1. Orbital | |||
| Globe -Bony erosions | 2( 13) | 3 (8 ) | 0.522 |
| Muscle infiltration | 10 (67) | 10 (26) | 0.005 |
| Fat infiltration | 14 (93) | 19 (49 ) | 0.003 |
| Optic Neuritis | 4 (27) | 2 (5) | 0.024 |
| Enhancement of orbital muscles | 5 (33) | 2 (5) | 0.006 |
| Enhancing soft tissue mass In Orbital Apex | 9 (60) | 2 (5) | 0.000 |
| Thickening and lateral displacement of inferior and medial rectus muscles. | 10 (67) | 9 (23 ) | 0.003 |
| Proptosis | 9 (60) | 8 (21 ) | 0.005 |
| Preseptal cellulitis | 8 (53) | 3 (7 ) | 0.000 |
| Prominent superior ophthalmic vein | 1 (7) | 1 (3) | 0.475 |
| 2. Paranasal Sinuses | |||
| Frontal sinus opacification and mucosal thickening | 13 (87) | 16 (41 ) | 0.003 |
| Maxillary sinus opacification and mucosal thickening | 15 (100) | 35 (90 ) | 0.197 |
| Ethmoid sinus opacification and mucosal thickening | 15 (100) | 35 (90 ) | 0.197 |
| Sphenoid sinus opacification and mucosal thickening | 15 (100) | 23 (59) | 0.003 |
| Cavernous sinus opacification and mucosal thickening | 14 (93) | 0 ( 0) | 0.000 |
| Infratemporal Fossa Infiltration | 11 (73) | 11 (28) | 0.003 |
| Air Fluid Level | 9 (60) | 7 (18) | 0.002 |
| Soft Tissue Infiltration of maxillary periantral fat planes | 14 (93) | 23 (59 ) | 0.015 |
| Bony Dehiscence | 9 (60) | 7 (18) | 0.002 |
| 3. Nose | |||
| Medial Concha infiltration | 4 (27) | 6 (15 ) | 0.339 |
| Inferior Concha infiltration | 7 (47) | 13 (33 ) | 0.574 |
| Inferior Turbinate Sign | 2 (13) | 2 (5) | 0.495 |
| Bony dehiscence | 9 (60) | 5 (13) | 0.002 |
| 4. Brain | |||
| Infarcts/Others( Abscesses, Edema) | 6 (40)/6 (40) | 11 (28 )/11 (28) | 0.403 |
| 5. Cavernous sinus-Direct Features | |||
| Convexity of lateral wall of Cavernous Sinus | 12 (80) | 0 ( 0) | |
| Abnormal signal intensity on T1/T2 images. | 13 (87) | 0 ( 0) | |
| Filling defects in the cavernous sinus | 9 (60) | 0 ( 0) | |
| Cavernous internal carotid artery stenosis | 7 (47) | 0 ( 0) |
Sphenoid and frontal sinus opacification and mucosal thickening, soft-tissue infiltration of maxillary periantral fat planes, involvement of infratemporal fossa, air fluid level in sinuses, and bony dehiscence of sinuses were significant findings in CSS-positive group. The direct qualitative features in CSS-positive group were abnormal signal intensity, convexity of lateral wall, filling defects, and internal carotid artery stenosis in 13 (87%), 12 (80%), 9 (60%), and 7 (47%), respectively. MR venogram could be done in 20% (n = 3) subjects which showed abnormal signal intensity in cavernous sinus and prominent superior ophthalmic vein [Table 3].
Management including surgical intervention with maxillectomy (n = 2 [13%]), orbital exenteration (n = 4 [27%]), and evisceration of eye (n = 3 [20%]) and use of intravenous liposomal or deoxycholate amphotericin-B and were performed significantly more in CSS-positive group. Clinical stability or regression of disease was seen in 53% (n = 8) and were discharged from the hospital. In CSS-positive group, mortality rate at 8 and 24 weeks was 13 and 27%, respectively [Table 1].
DISCUSSION
This study showed CSS in 28% (n = 15) of COVID-19-associated ROCM similar to the previous reports.[17,18] COVID-19 pandemic witnessed an exponential increase in coexisting infection with ROCM and extension to brain significantly affected the outcome.[9,10,19] This study showed significant differences in clinical features of CSS-positive as compared to CSS-negative group including age of presentation, ocular, nasal, systemic features, cranial nerve involvement, and radiological findings in the orbit, paranasal sinuses, nose, and brain. Recognition of these features can help in early clinical prediction of spread of infection to the brain.
Patients can have ROCM during and even up to 3 months after recovery from COVID-19 and in this study, a higher number of CSS-positive group had active COVID-19 status.[20,21] Most of the subjects in CSS-positive group were proven and in advanced stages of ROCM as compared to CSS-negative group [Table 1]. The probable reason was aggressiveness of infection due to comorbidities and alterations in immunity during COVID-19.[9,18] As per our knowledge, the previous studies have not used the objective categorization and staging systems to assess the severity of CSS and ROCM.
CSS-positive group was significantly younger than CSS-negative group as seen in recent studies as compared to pre-COVID-19 era.[22,23] Males were affected more than females as consistent with the previous studies because COVID-19 and ROCM was more common in males, possibly due to outdoor exposure.[9,10,17,24] However, CSS due to non-infective causes was more commonly seen in females.[12] In our study, majority of the subjects were not vaccinated which emphasizes the role of COVID-19 vaccination to mitigate the risk of such coinfections. The duration of symptoms was significantly longer in CSS-positive group as seen previously.[9,10]
Common risk factors associated with CSS and ROCM were diabetes mellitus and use of corticosteroids as reported recently.[9,24] Intravenous corticosteroid used for immune modulation during COVID-19 causes hyperglycemia leading to impaired chemotaxis and phagocytosis of neutrophils. This along with excessive availability of free iron in diabetic ketoacidosis and decreased number of lymphocytes in coexisting COVID-19 increases the propensity to invasive ROCM. Other immunodeficiency states in this study group were primary or secondary immunodeficiency and organ transplantation similar to a recent study.[9]
CSS-positive group had increased features of ocular, nasal, and cerebral system involvement [Table 2]. Ocular features in CSS were more as compared to previously reported presentations with ROCM and concurrent COVID-19.[7,17,16] Eyelid, periocular and facial discoloration, ptosis, proptosis, and ophthalmoplegia were significantly more as compared to CSS-negative ROCM group which had been reported in a variable range.[10,24] These differences may be attributed to concurrent COVID-19 and variation in clinical presentations across these studies. Vision loss was seen in 73% subjects in our study as compared to previously reported 25–80% with ROCM.[7,25] Visual acuity was affected less in CSS due to non-infective etiology as compared to those with COVID-19-associated ROCM which may be attributed to perineural and perivascular spread of infection to optic nerve.[9,12]
ROCM spreads from nose and paranasal sinuses through lamina papyracea and orbital fissures to apex of orbit which acts as a window to cavernous sinus.[26] Our study showed that orbital apex lesion was more in CSS-positive as compared to CSS-negative group as had been seen in recent studies.[12,16] CSS-positive group had significant indirect radiological features including orbital and muscle infiltration, proptosis, pre-septal cellulitis, enhancement of orbital muscles, and optic neuritis which were more than that reported previously.[16] Clinicoradiological diagnosis of orbital apex syndrome can be useful in early recognition of the involvement of cavernous sinus in ROCM.
CSS-positive group had significant nasal discharge and mucosal inflammation as had been noted in a recent multicentric study from India.[9] Regional pain in paranasal sinus, orbit, or teeth in CSS may be due to fifth cranial nerve involvement or invasion of blood vessels in the bone by mucormycosis. In a recent study, dental pain was a common presentation of ROCM to oromaxillary facial surgeons during the COVID-19 pandemic.[7,24,27] Inferior turbinate sign and nasal bone dehiscence were more as compared to the previous studies probably due to more aggressiveness of infections.[9]
Cavernous sinus was more likely to be involved with extension of infection to adjoining sphenoid sinus.[10] Other paranasal sinuses including maxillary and ethmoid sinus showed opacification and mucosal thickening similar to another study.[16] However, infiltration of periantral fat planes and air fluid level in maxillary sinus and infratemporal fossa with bony dehiscence was significantly more in CSS-positive group than that reported earlier.[16]
Systemic features including fever were seen in all CSS-positive group and were significantly more as compared to CSS-negative group. The previous studies have reported fever in less than one-third of subjects, possibly due to inclusion of non-infective causes of CSS.[7,12,24] Cerebral features such as headache, facial pain, paresthesias, and facial palsy were more in CSS-positive ROCM as compared to the previous studies.[24,10]
Most common cranial nerve involved in CSS-positive group were oculomotor, abducens, trochlear, and trigeminal nerve as opposed to another study where sixth nerve was followed by third, fourth, and fifth cranial nerve involvement [Table 2].[12] There is paucity of data regarding detailed description of involvement of cranial nerves in CSS associated with ROCM. Detailed neurological examination of cranial nerves is indispensable in diagnosing a clinical entity like CSS.
Due to higher risk of coinfection with ROCM in COVID-19, neuroimaging was performed more as compared to similar studies from India.[9,16] MRI brain is more sensitive for detecting ocular, paranasal sinuses, and brain involvement in ROCM. Whole cavernous sinus involvement was more common in contrast to a study from India which showed posterior cavernous sinus lesion, being most common in fungal infection.[12] The reason for this may be the aggressiveness of mucormycosis to involve the whole cavernous sinus with coexisting COVID-19 infection [Figure 2].

- (a) Clinical picture of a patient with cavernous sinus syndrome with COVID-19-associated ROCM showing proptosis, ptosis, and ophthalmoplegia. (b) Axial view of CT head showing proptosis (red arrow), soft-tissue thickening in the right maxillary sinus (blue arrow), and soft-tissue thickening in the right temporoparietal region extending to scalp (yellow arrow). (c) Exenterated eye of patient with cavernous sinus syndrome with COVID-19-associated ROCM showing growth of mucormycosis (black arrow). (d) Axial view of contrast enhanced T1W MRI image of an exenterated eye showing non-enhancing right cavernous sinus (red arrow) and right cavernous ICA stenosis compared to the left side (black arrow).
In our study, direct qualitative neuroimaging features in CSS-positive group included abnormal signal intensity and convexity of lateral wall of cavernous sinus as reported previously [Table 3].[14,15] Cavernous sinus thrombosis and internal carotid artery involvement were more as compared to the previous studies.[16,18,24] These vascular complications were due to spread of infection through adjoining orbits or sinuses or veins and hypercoagulable state with COVID-19.
Proven ROCM with microbiological evidence of mucormycosis in CSS-positive group was similar to a previous study.[24] Treatment of ROCM included antifungals and surgical intervention depending on clinical presentation, radiological extension, and tissue diagnosis. CSS-positive with proven ROCM was treated with intravenous liposomal or deoxycholate amphotericin-B similar to another study.[9] Treatment with oral posaconazole was given to all CSS (all categories of ROCM) due to high index of suspicion, coexistent comorbidities, and limited supply of Amphotericin B.
Surgical treatment was performed significantly more in CSS-positive group due to a higher number of subjects being advanced stages of proven ROCM. Although the literature shows that surgical intervention in ROCM with extension to CNS did not affect the survival but few studies have shown the positive effect on outcome.[9,28] The combined therapy of antifungal along with surgical intervention had better prognosis than either of them alone in ROCM but data specific to involvement of cavernous sinus are lacking.[9] Our study did not show significant difference in outcome across both the groups. The mortality rate of ROCM with orbital and cerebral involvement may be 30–90% but during the COVID-19 pandemic, it decreased to 14–40%.[9,22,29] The lower mortality rate in our study can be explained by high index of suspicion and early diagnosis of ROCM and CSS in admitted patients during the second peak of COVID-19. In view of the prevailing COVID-19 pandemic with newer strains and rising fungal infections, further multicenter research involving a larger population is needed to understand the disease.
The limitations of the study are small sample size and a single-center experience. There were limited enrollment and follow-up of patients due to relocation of patients elsewhere possibly due to concurrent COVID-19 pandemic. Since it was a hospital-based study, subjects with milder disease may not have been admitted. The strength of study is prospective design and since the medical fraternity was novice to COVID-19, this study shows how COVID-19-associated ROCM unfolded itself to involve cavernous sinus and brain. Moreover, the study used objective clinicoradiological methods to categorize and stage both ROCM and CSS.
CONCLUSION
Incidence of CSS with COVID-19-associated ROCM was 28%. Extension of ROCM to CSS was more common in young males in advanced stages of proven ROCM associated with concurrent COVID-19. CSS-positive group had significant difference in clinicoradiological features involving orbit, nose, paranasal sinuses, and central nervous system as compared to CSS-negative group. This study highlights the need for further development and validation of an objective scoring system considering clinical and radiological features for early diagnosis of CSS in a larger sample of population across multicenter.
Ethical approval
The study was approved by the Institutional Ethics Committee-GGS/IEC/20/107.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ANNEXURE
| Nasal features | Ocular features | Systemic and cerebral features |
|---|---|---|
| Nasal Stuffiness | Proptosis | Fever |
| Foul Smell | Loss of vision | Headache |
| Epistaxis | Ophthalmoplegia | Facial Pain |
| Nasal Discharge | Ptosis | Facial Paresthesia, Anesthesia |
| Nasal Mucosal Erythema, Inflammation, Purple or Blue Discoloration, White Ulcer, Ischemia, or Eschar | Eyelid Periocular or Facial Edema | Facial Palsy |
| Regional Pain-Orbit, Paranasal Sinus or Dental Pain | Eyelid, Periocular, Facial Discoloration | Altered Sensorium, Paralysis, Focal Seizures |
| Direct radiological features | Indirect radiological features | ||
|---|---|---|---|
| Cavernous sinus | Orbit | Sinus | Nose |
| 1. Convexity of Lateral Wall of Cavernous Sinus 2. Abnormal Signal Intensity on T1/T2 3. Filling Defects in Cavernous Sinus 4. Cavernous ICA stenosis |
1. Globe -Bony Erosions 2. Muscle Infiltration 3. Fat Infiltration 4. Optic Neuritis 5. Enhancement of Orbital Muscles 6. Enhancing Soft Tissue Mass In Orbital Apex 7. Thickening and Lateral Displacement of Inferior and Medial Rectus 8. Proptosis 9. Preseptal Cellulitis 10. Prominent Superior Ophthalmic Vein |
1. Sinus Opacification and Mucosal Thickening in : Frontal , Maxillary, Ethmoid , Sphenoid, Cavernous sinus 2. Infratemporal Fossa Infiltration 3.Air Fluid Level 4. Soft Tissue Infiltration of Maxillary Periantral Fat Planes 5. Bony Dehiscence |
1. Medial Concha Infiltration 2.Inferior Concha Infiltration 3.Inferior Turbinate Sign 4. Bony Dehiscence |
| Anterior CSS | Middle CSS | Posterior CSS | Whole CSS |
|---|---|---|---|
| Optic neuropathy or isolated palsy of superior or inferior branch of Oculomotor nerve, regardless of other ocular motor nerves or Ophthalmic nerve involvement | Concurrent oculomotor nerve and ophthalmic nerve involvement | Involving the maxillary nerve or abducens nerve with Horner’s Syndrome | Involving both optic nerve and maxillary nerve in addition to ocular motor nerves and ophthalmic nerve involvement |
References
- Epidemiology and diagnosis of mucormycosis: An update. J Fungi. 2020;6:265.
- [CrossRef] [PubMed] [Google Scholar]
- Mucor in a viral land: A tale of two pathogens. Indian J Ophthalmol. 2021;69:244-52.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69:1002-4.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459-68.
- [CrossRef] [PubMed] [Google Scholar]
- Rhinocerebral mucormycosis: Evolution of the disease and treatment options. Laryngoscope. 1997;107:855-62.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features, diagnosis, and outcomes of rhino-orbito-cerebral mucormycosis-a retrospective analysis. Curr Med Mycol. 2016;2:15-23.
- [CrossRef] [PubMed] [Google Scholar]
- Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol. 1994;39:3-22.
- [CrossRef] [PubMed] [Google Scholar]
- Bilateral cavernous sinus thrombosis complicating sinusitis. J R Soc Med. 2006;99:474-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India-Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69:1670-92.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentations, management and outcomes of rhino-orbital-cerebral mucormycosis (ROCM) following COVID-19: A multi-centric study. Ophthalmic Plast Reconstr Surg. 2021;37:488-95.
- [CrossRef] [PubMed] [Google Scholar]
- Cavernous sinus syndrome: Clinical features and differential diagnosis with MR imaging. AJR Am J Roentgenol. 2003;181:583-90.
- [CrossRef] [PubMed] [Google Scholar]
- Which classification of cavernous sinus syndrome is better-Ishikawa or Jefferson? A prospective study of 73 patients. J Neurosci Rural Pract. 2016;7(Suppl1):S68-71.
- [CrossRef] [PubMed] [Google Scholar]
- Code mucor: Guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69:1361-5.
- [CrossRef] [PubMed] [Google Scholar]
- The Ishikawa classification of cavernous sinus lesions by clinico-anatomical findings. Jpn J Ophthalmol. 2001;45:420-4.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of cavernous sinus thrombosis. Eur J Radiol Open. 2020;7:100226.
- [CrossRef] [PubMed] [Google Scholar]
- A multicentric observational study of imaging findings in COVID-19-related rhino-orbito-cerebral mucormycosis: A new Pandora's box. Egypt J Radiol Nucl Med. 2021;52:258.
- [CrossRef] [Google Scholar]
- Predictive factors for invasive fungal rhinosinusitis in diabetic patients: Systematic review and data re-analysis. Asian Pac J Allergy Immunol. 2021;39:1-8.
- [Google Scholar]
- Definition and management of invasive fungal rhinosinusitis: A single-centre retrospective study. Acta Otorhinolaryngol Ital. 2021;41:43-50.
- [CrossRef] [PubMed] [Google Scholar]
- Rhino-orbito-cerebral mycosis and COVID-19: From bad to worse? Ann Indian Acad Neurol. 2022;25:68-75.
- [CrossRef] [PubMed] [Google Scholar]
- Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135:442-7.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15:102146.
- [CrossRef] [PubMed] [Google Scholar]
- Outbreak of mucormycosis in coronavirus disease patients, Pune, India. Emerg Infect Dis. 2022;28:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging features of rhinocerebral mucormycosis: A study of 43 patients. Egypt J Radiol Nucl Med. 2018;49:447-52.
- [CrossRef] [Google Scholar]
- Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670-4.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of rhino-orbitocerebral mucormycosis (phycomycosis): A report of 16 personally observed cases. Ophthalmology. 1983;90:1096-104.
- [CrossRef] [PubMed] [Google Scholar]
- Orbital exenteration in rhino-orbito-cerebral mucormycosis: A prospective analytical study with scoring system. Indian J Otolaryngol Head Neck Surg. 2019;71:259-65.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 associated mucormycosis (CAM) in India: A formidable challenge. Br J Oral Maxillofac Surg. 2021;59:1095-8.
- [CrossRef] [PubMed] [Google Scholar]
- The emergence of COVID-19 associated mucormycosis: A review of cases from 18 countries. Lancet Microbe. 2022;3:e543-52.
- [CrossRef] [PubMed] [Google Scholar]
- A case series of invasive mucormycosis in patients with COVID-19 infection. Int J Otorhinolaryngol Head Neck Surg. 2021;7:867-70.
- [CrossRef] [Google Scholar]






