Translate this page into:
Magnetic Resonance Imaging Evaluation of Perinatal Hypoxic Ischemic Encephalopathy: An Institutional Experience
Ravikanth Reddy, MD, DNB, EDiR, FRCR Department of Radiology, St. John's Hospital Karnataka 560034 India ravikanthreddy06@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background Hypoxic–ischemic encephalopathy (HIE) is the most commonly diagnosed neurological abnormality affecting children leading to severe neurological deficits and a cause of neonatal mortality. HIE constitutes a diagnostic challenge in the prematurely born and full-term neonates. HIE causes severe neurological deficit in children and many a times goes unnoticed in early stages. The various patterns of central nervous system (CNS) involvement in HIE are dependent on factors, such as severity and duration of hypoxia, and brain maturity in preterm and full-term patients. Magnetic resonance imaging (MRI) has prognostic significance in detecting patterns of HIE secondary to mild-to-moderate and severe hypoxias and the imaging findings are highly dependent on the time at which imaging is done. MRI helps determine the prognosis of brain development in patients with HIE.
Objective This retrospective study elucidates the spectrum of MRI findings in preterm and full-term patients with HIE on MRI.
Materials and Methods This retrospective descriptive study was conducted at a tertiary care center between April 2017 and May 2019 on 50 patients with a clinical diagnosis of HIE using a General Electric (GE) 1.5-Tesla MRI scanner. Various patterns of HIE were evaluated on MRI in preterm and full-term patients.
Results This retrospective study evaluated MRI findings in 50 infants diagnosed with HIE. Eighteen (36%) were preterm and 32 (64%) were full-term patients. Thirty-five (70%) were male and 15 (30%) were female patients. In the current study, developmental delay was the most commonly associated clinical entity in both preterm and full-term patients. In preterm patients, periventricular leukomalacia was the most prevalent MRI finding, and in full-term patients, subcortical and periventricular white matter hyperintensities on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences were most commonly encountered.
Conclusion MRI is the primary imaging modality of choice in preterm and full-term patients with HIE, as it helps determine the severity of hypoxic–ischemic injury by understanding the pattern of brain involvement. In the current study, distinguishable patterns of MRI findings secondary to birth asphyxia and ischemic insult were elucidated in both preterm and full-term patients who are highly dependent on the level of brain maturity at the time of imaging. Regular MRI follow-up has a prognostic significance in HIE with accurate prediction of neurodevelopmental outcome on follow-up studies.
Keywords
germinal matrix hemorrhage
hypoxic–ischemic injury
hypoxic–ischemic encephalopathy
periventricular leukomalacia
thinning of corpus callosum
Introduction
Perinatal asphyxia occurs secondary to reduced oxygen supply causing ischemia, with resultant permanent damage to the brain. Hypoxic–ischemic encephalopathy (HIE) refers to hypoxia-induced perinatal insult causing ischemic injury to the brain tissue within a time frame of 12 to 36 hours, leading to neonatal encephalopathy.1 Perinatal asphyxia occurs due to the synergistic effect of hypoxia and hypercarbia leading to metabolic acidosis which is the underlying mechanism of injury in HIE. Insufficient cerebral ventilation–perfusion combination causes cerebral vascular dysregulation. HIE occurs due to fetoplacental insufficiency or umbilical vessel occlusion secondary to birth asphyxia. Pathophysiology of HIE is related to series of occurrences leading to severe neurological damage during the perinatal period. The incidence of HIE is approximately 7 in 1,000 preterm and 2.5 in 1,000 full-term live births.2 Global trends of HIE-related neonatal deaths due to nonspecific conditions vary between 0.7 and 1.6 million live births per year. In Southeast Asia, prenatal asphyxia has an incidence of approximately 3.3% and mostly related to low birth weight and preterm gestational age.3 In infants with severe hypoxia, mortality rate stands at 25 to 50% during the neonatal period with approximately 40% of deaths occurring below the age of 5 years. Hypoxic–ischemic injury–related severe neurological damage has a prevalence rate of 25% in affected full-term neonates which accounts for 23% of neonatal deaths worldwide.4
Diagnosis of HIE is based on clinical evidence of fetal distress with poor Apgar's score at birth ranging between 0 and 3 at 5 minutes, necessity for resuscitation, low umbilical cord pH of <7.1 (acidosis), and a constellation of neurological abnormalities such as hypotonia, seizures, coma, and lastly immunodepressed state of multiorgan dysfunction syndrome (MODS).2 HIE causes lasting permanent damage to the infant brain having a major implications on the child, family, and socioeconomic impact on the society at large. On magnetic resonance imaging (MRI), the spectrums of findings in HIE are not consistent and are dependent on factors, such as severity and duration of birth asphyxia, and the level of brain maturity. Patterns of hypoxic–ischemic injury in preterm neonates (<37 weeks' gestational age) are apparent and distinguishable from that of full-term neonates (≥37 weeks' gestational age). Also, the patterns of brain injury in mild-to-moderate and severe hypoxias are distinct.5 Subtle lesions that may not be easily identifiable on ultrasonography, such as central lesions in the thalamus, hippocampus, brain stem, and cerebellum, bilateral parasagittal lesions and cortical/subcortical based lesions may be easily diagnosed with precision and accuracy on MRI.6 Treatment in hypoxic–ischemic injury to the brain is mostly conservative and primarily aims at restraining the extent of neurological deficits. MRI has significant role in the early diagnosis of HIE by evaluating the extensiveness and severity of perinatal insult, predicting prognosis, and for planning therapeutic strategies.
Materials and Methods
Setting
This retrospective descriptive study was conducted at a tertiary care center between April 2017 to May 2019 on 50 patients diagnosed with HIE using a GE 1.5-Tesla MRI scanner (Signa, General Electric Medical Systems, Milwaukee, Wisconsin, United States). The current study elucidates the spectrum of MRI findings in preterm and full-term patients with HIE on MRI.
Patients and Design
All patients included were in the pediatric age group (0–5 years) with history of perinatal birth asphyxia and clinical findings suggestive of hypoxic–ischemic injury. MRI brain was performed on these patients as part of the protocol and various patterns of HIE in both preterm and full-term patients were evaluated. Patients with persistent clinical signs of a floppy infant, such as bradycardia, hypotonia, poor feeding, seizures, and depressed level of consciousness, were included in the current study. Seizures, hemiplegia/paraplegia/quadriplegia, developmental delay, and cerebral palsy were some of the clinical criteria used for assessment of patients on MRI sequences.
Magnetic Resonance Imaging
Axial T1-weighted and T2-weighted sequences, axial diffusion-weighted imaging (DWI) with corresponding apparent diffusion coefficient (ADC) mapping, coronal and sagittal T2-weighted sequences, axial T2 fluid-attenuated inversion recovery (FLAIR) sequences, and susceptibility-weighted imaging (SWI) were part of the protocol while performing MRI brain in patients. Three-dimensional (3D) sequences, such as T2 SPACE sequence and MP-RAGE (Magnetization Prepared – RApid Gradient Echo sequence), were used as appropriate. Longer T1 and T2 relaxation times of MR sequences have been adapted for use, while imaging the neonatal brain as opposed to the standard MR sequences was used for image acquisition due to higher proportion of water and lower proportion of proteins and lipids in the neonatal brain. This is achieved by increasing the repetition time (TR) of T1-weighted and T2-weighted MR sequences. Increasing the TR to 800 ms from the standard TR of 400 ms while acquiring T1-weighted images and increasing the TR to 6,500 ms from the standard TR of 4,000 ms while acquiring T2-weighted images were recommended during image acquisition of MR sequences.7
Axial T1-weighted images are ideal for evaluating myelination, demyelination, dysmyelination, and subacute hemorrhage. Axial T2-weighted images are ideal for mapping out white matter signal changes, as T2-weighted MRI sequence displays sharp contrast between gray matter and white matter. Gradient echo T2* (T2-star) or SWI MR sequence is optimal for indicating hemorrhage and for discriminating hemorrhage from ischemic and gliotic foci. As compared with conventional T1- or T2-weighted sequences, DWI with corresponding ADC mapping done between 1 and 7 days of postnatal period is ideal for the identification of cytotoxic edema. DWI sequence performed in the perinatal period is extremely good for the spotting damage to white matter. Acute ischemic regions show restricted diffusion which is demonstrated as hyperintense signal intensity changes on DWI sequence with corresponding hypointensity on ADC mapping, and the DWI hyperintensity generally peaks 3 to 5 days after the hypoxic–ischemic insult which eventually normalizes regardless of ongoing neuronal injury.2
FLAIR sequence is particularly useful for perception of white matter abnormalities which manifests as hyperintense signal intensity changes on T2-weighted MR sequence consistent with cystic encephalomalacia and gliosis. After completion of myelination, the FLAIR sequence is optimal for exemplifying cortical gliosis and periventricular leucomalacia.8 For evaluating HIE in the postnatal period, DWI is the ideal imaging sequence in the first week of postnatal period, and conventional T1- and T2-weighted sequences are diagnostically most accurate sequences from the second week of postnatal period. The optimal time frame for MRI evaluation of HIE and perinatal asphyxia is between 5 and 14 days in the postnatal period.9
Data Analysis and Statistics
The current study performed a descriptive analysis of imaging findings on MRI in preterm and full-term infants with HIE. The collected data were tabulated using Microsoft Excel 2010 (Microsoft Corp., Redmond, Washington, United States), and statistical analyses were conducted using SPSS Statistical Package (version 20.0), IBM SPSS Statistics for Windows, V.20.0 (IBM Corp., Armonk, New York, United States).
Ethical Considerations
All examinations performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional Ethics Committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from parent(s)/guardian(s) prior to the enrollment of patients in this study.
Results
Sex Wise Distribution of Patients
Among 50 patients included in the study, 35 were males and 15 were females. The affected male-to-female sex ratio in this study is 2.3:1.
Age Wise Distribution of Patients
The age wise distribution of patients ranged between 0 to 5 years. Distribution of spectrum of MRI findings were most commonly encountered in the age group of 1 to 5 years.
Distribution Pattern of Magnetic Resonance Imaging Findings in Term Patients
Thirty-two full-term patients with history of perinatal hypoxia and a suspicion of HIE underwent MRI brain. Twenty-five (78.1%) patients demonstrated hyperintense white matter signal intensity change which is the most prevalent finding on T2 and FLAIR MR sequences. Additional findings reported were acute infarcts, delayed myelination, cystic encephalomalacia, cerebral cortical atrophy, and corpus callosum thinning (Table 1).
|
Distribution pattern of MRI findings |
No. of cases/total full-term patients |
Percentage |
|---|---|---|
|
T2 and FLAIR white matter hyperintensities |
25/32 |
78.1 |
|
Cerebral cortical atrophy |
20/32 |
62.5 |
|
Encephalomalacia |
15/32 |
46.8 |
|
Delayed myelination |
15/32 |
46.8 |
|
Thinning of corpus callosum |
13/32 |
40.6 |
|
Basal ganglia and thalami |
8/32 |
25.0 |
|
Acute ischemic infarcts |
3/32 |
1.2 |
|
Perirolandic hyperintensities |
2/32 |
6.2 |
Abbreviations: FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging.
Distribution Pattern of Magnetic Resonance Imaging Findings in Preterm Patients
Eighteen preterm patients who had a history of birth asphyxia were assessed in the current study on MRI sequences. The most prevalent findings on MRI in preterm patients were cerebral atrophy which constituted the majority is seen in 11 of 18 (61.1%) followed by periventricular leukomalacia which is seen in 10 of 18 (55.56%). Additional findings encountered were germinal matrix hemorrhage, delayed myelination, acute infarcts, and corpus callosum thinning (Table 2).
|
Distribution pattern of MRI findings |
No. of cases/total preterm patients |
Percentage |
|---|---|---|
|
Cerebral cortical atrophy |
11/18 |
61.1 |
|
Periventricular leucomalacia |
10/18 |
55.5 |
|
Thinning of corpus callosum |
9/18 |
50.0 |
|
Delayed myelination |
8/18 |
44.4 |
|
Acute ischemic infarcts |
3/18 |
16.6 |
|
Germinal matrix hemorrhage |
3/18 |
16.6 |
Abbreviation: MRI, magnetic resonance imaging.
Distribution Pattern of Magnetic Resonance Imaging Findings in Patients with Cerebral Palsy
Among 50 evaluated patients of HIE, 8 had clinical features suggestive of cerebral palsy; three of eight (37.5%) constituting preterm and five of eight (62.5%) being full-term patients. In patients with cerebral palsy, cerebral cortical atrophy is the most prevalent imaging finding overall. In preterm patients with cerebral palsy, periventricular leukomalacia is the most prevalent imaging finding and in term neonates; hyperintense lesions on T2 and FLAIR sequences are the most common finding. Additional findings encountered were delayed myelination, cystic encephalomalacia, and thinned out corpus callosum (Table 3).
|
Distribution pattern of MRI findings |
No. of cases/total patients with cerebral palsy |
Percentage |
|---|---|---|
|
Cerebral cortical atrophy |
7/8 |
87.5 |
|
T2 and FLAIR white matter hyperintensities |
6/8 |
75.0 |
|
Thinning of corpus callosum |
5/8 |
62.5 |
|
Delayed myelination |
4/8 |
50.0 |
|
Cystic encephalomalacia |
2/8 |
25.0 |
|
Periventricular leucomalacia |
2/8 |
25.0 |
Abbreviations: FLAIR, fluid-attenuated inversion recovery; MRI, magnetic resonance imaging.
Gestational Age Wise Distribution of Patients with Hypoxic–Ischemic Encephalopathy
In the current study of 50 infants with clinical features of HIE, 18 were preterm and 32 were full term (Table 4).
|
Age (postconceptional in weeks) |
Mild–moderate injury |
Severe injury |
|---|---|---|
|
Premature neonates (<37 weeks) |
Periventricular white matter hyperintensities and germinal matrix hemorrhage |
Anterior vermis, thalami and dorsal brain stem are more frequently affected than basal ganglia |
|
Term neonates (>37 weeks) |
Parasagittal watershed regions and subcortical white matter |
Dorsal brain stem, ventrolateral thalami, basal ganglia, lateral geniculate nuclei, hippocampi and sensorimotor cortex |
Discussion
HIE has remained a challenge to the medical fraternity, especially in the developing nations and third-world countries with limited resources. HIE continues to make its mark as the most common cause of neurodevelopmental delay, morbidity, and mortality in many countries despite major advances in medicine which include better understanding of HIE pathophysiology. HIE presents with complications such as permanent long-term brain damage. Hence early diagnosis with planning of therapeutic strategies to minimize the after effects and damage control remains the mainstay of management in HIE. Advanced imaging techniques and newer MRI sequences can have prognostic implications by early identification of subtle lesions which may be missed on neurosonograms.
Normal Magnetic Resonance Imaging Maturation Appearances of Neonatal Brain
The degree and extent of myelination is a marker of brain maturation and the signal intensities of myelinated and unmyelinated white matter on MRI sequences of the relatively immature neonatal brain vary from that of adult brain. Myelinated white matter in adults appears as high signal intensity on T1-weighted images and as low signal intensity on T2-weighted images as compared with the gray matter. In a fetus, myelination begins at 6 weeks intrapartum and is almost complete by the postnatal age of 2 years. In full-term neonates, myelinated white matter demonstrates increased signal intensity on T1-weighted images and is seen in the ventrolateral part of the thalamus and posterior limb of the internal capsule. Therefore, in a normal neonatal brain, there is reversal of cortical gray or white matter signal on MR sequences.10
Patterns of Brain Injury in Hypoxic–Ischemic Encephalopathy
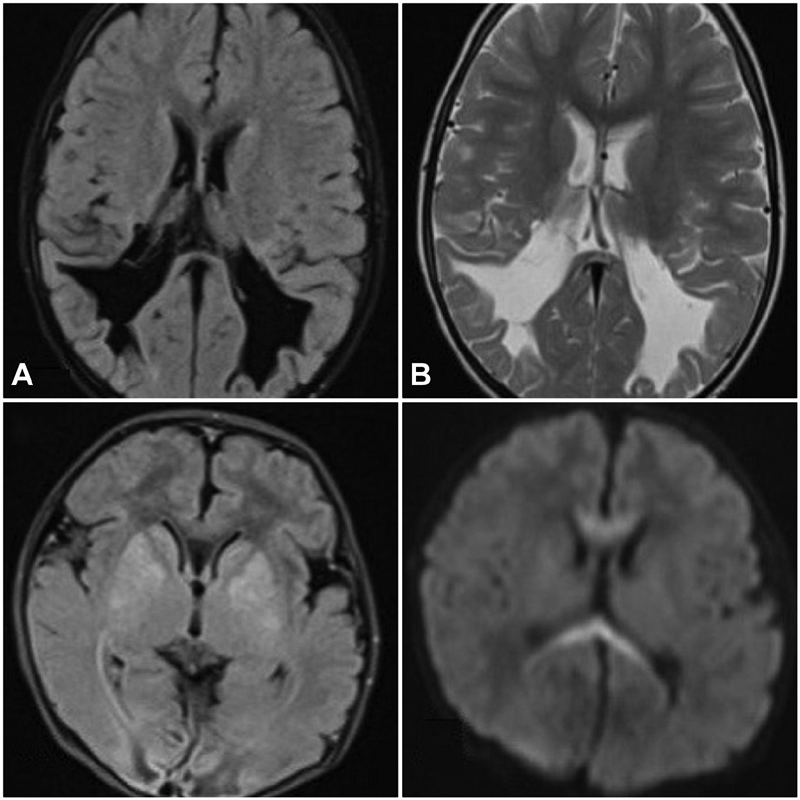
Cortical, subcortical and periventricular white matter, basal ganglia, internal capsule and thalami, and medial temporal lobes are the most prevalent affected regions of brain in HIE. To induce brain damage in the perinatal period, a hypoxic–ischemic insult lasting longer than 10 to 15 minutes would be adequate. The patterns of asphyxia-related brain injury in HIE are dependent of factors such as severity and duration of the hypoxic–ischemic event, time of insult during the perinatal period, and also on the level of brain maturity (Figs. 1 and 2).

-
Fig. 1 Upper panel figures (A–D) A 2-month-old infant with history of birth asphyxia and clinical features of hypoxic–ischemic encephalopathy. Axial T2 FLAIR MR image at the supraganglionic level (A) and axial T2 FLAIR MR image at the ganglionic level (B) demonstrating periventricular and subcortical white matter hyperintensities, leucomalacia, cortical thinning with dilated sulcal spaces in bilateral frontoparietal lobes suggesting gliosis with encephalomalacia changes. Axial diffusion-weighted MR image (C) shows unrestricted diffusion and sagittal T2 FLAIR MR image (D) demonstrates thinned out corpus callosum. Lower panel figures (E–H): axial T2 FLAIR (E) and axial T2-weighted (F) MR images demonstrating gliosis with cystic encephalomalacia changes in a 4-month-old infant. Axial diffusion-weighted (G) and apparent diffusion coefficient (ADC) (H) MR images demonstrating deep white matter hyperintense area adjacent to the right lateral ventricle with corresponding hypointensity on ADC map suggesting acute ischemic infarction. FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance.
Fig. 1 Upper panel figures (A–D) A 2-month-old infant with history of birth asphyxia and clinical features of hypoxic–ischemic encephalopathy. Axial T2 FLAIR MR image at the supraganglionic level (A) and axial T2 FLAIR MR image at the ganglionic level (B) demonstrating periventricular and subcortical white matter hyperintensities, leucomalacia, cortical thinning with dilated sulcal spaces in bilateral frontoparietal lobes suggesting gliosis with encephalomalacia changes. Axial diffusion-weighted MR image (C) shows unrestricted diffusion and sagittal T2 FLAIR MR image (D) demonstrates thinned out corpus callosum. Lower panel figures (E–H): axial T2 FLAIR (E) and axial T2-weighted (F) MR images demonstrating gliosis with cystic encephalomalacia changes in a 4-month-old infant. Axial diffusion-weighted (G) and apparent diffusion coefficient (ADC) (H) MR images demonstrating deep white matter hyperintense area adjacent to the right lateral ventricle with corresponding hypointensity on ADC map suggesting acute ischemic infarction. FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance.
-
Fig. 2 Upper panel figures (A–B): a 3-month-old infant with history of birth asphyxia demonstrating periventricular leucomalacia. Axial T2 FLAIR MR image (A) and axial T2-weighted MR image (B) at the ventricular body level demonstrating paucity of periventricular and deep white matter in bilateral occipital lobes with ex-vacuo dilatation of posterior horns of lateral ventricles. Lower panel figures (C and D): a 5-month-old infant with history of preterm delivery and birth asphyxia. Axial T1-weighted MR image (C) demonstrating intrinsic hypointensity and axial diffusion-weighted MR image (D) demonstrating diffusion restriction in the corpus callosum (boomerang sign) suggesting acute ischemic infarction. FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance.
Fig. 2 Upper panel figures (A–B): a 3-month-old infant with history of birth asphyxia demonstrating periventricular leucomalacia. Axial T2 FLAIR MR image (A) and axial T2-weighted MR image (B) at the ventricular body level demonstrating paucity of periventricular and deep white matter in bilateral occipital lobes with ex-vacuo dilatation of posterior horns of lateral ventricles. Lower panel figures (C and D): a 5-month-old infant with history of preterm delivery and birth asphyxia. Axial T1-weighted MR image (C) demonstrating intrinsic hypointensity and axial diffusion-weighted MR image (D) demonstrating diffusion restriction in the corpus callosum (boomerang sign) suggesting acute ischemic infarction. FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance.
Mild-to-Moderate Hypoxic–Ischemic Encephalopathy
In mild-to-moderate hypoperfusion, autoregulation protects the brain by causing blood flow to redistribute to the metabolically hyperactive deep gray matter structures of the brain, thus sparing the brain stem, basal ganglia, and thalami. The interarterial watershed zones of the cerebrum are prone for injury in mild-to-moderate hypoperfusion. As the brain matures, the vascular supply to the brain is altered. In preterm neonates, hypoperfusion leads to ischemic injury to the cerebral white matter at the periventricular border zone and in full-term neonates, hypoperfusion causes injury to cortical parasagittal and subcortical white matter.
Severe Brain Hypoxic–Ischemic Encephalopathy
However, there is complete loss of autoregulation in severe hypoperfusion to the brain. Deep gray matter nuclei, such as the brain stem and thalami, and actively myelinating fibers are particularly vulnerable to severe hypoperfusion injury. Severe hypoxic ischemia preferentially affects the thalami, dorsal brainstem, and anterior vermis with relative sparing of the cortex and deep gray matter structures in preterm neonates which is in contrast to full-term neonates where severe hypoxic ischemia particularly affects the ventrolateral thalami, dorsal brainstem, hippocampi, posterior putamina, and occasional involvement of the sensorimotor cortex.
In the current retrospective study, fifty patients with history of perinatal birth asphyxia and clinical features of HIE were studied on MRI sequences. Thirty-five (70%) male and fifteen (30%) female patients were included. The sex ratio (male:female) is 2.3:1 in the current study which is consistent with the finding in the study performed by Lakhkar et al11 which had a 3:1 male preponderance. In a study done by Qureshi et al,12 the sex ratio is 3.9:1 with 79.6% male and 20.4% female patients.
In the current study, the age group of patients ranged between 0 and 5 years. In a study performed by Connolly et al,13 the range of age groups of patients considered were between 1 and 24 years with majority patients in the age group of 1 to 5 years which is consistent with the current study.
The current study included 18 (36%) preterm and 32 (64%) full-term patients which correlates with the findings by Yin et al14 that included a total of 42 patients among which 12 were premature (28.5%) and 30 were full-term (71. 4%). Similarly, in a study performed by Qureshi et al3 on 181 patients, 19.1% were premature and 77.9% were full-term which is consistent with the current study.
In the current study, 25 (78.1%) patients had cortical and subcortical hyperintensities on T2 and FLAIR sequences and 20 (62.5%) had cerebral cortical atrophy. In patients with acute symptoms, 4 (1.2%) had acute infarcts and 15 (46.8%) had delayed myelination. In a study done by Rutherford et al15 with HIE patients, the clinical outcome correlated well with MR findings. The findings in the study were cortical and subcortical hyperintensities on T2-weighted images in 50% of cases and cerebral cortical atrophy in the rest 50% of cases. In patients with acute symptoms, 18% cases had basal ganglia infarcts, 6% cases had insular infarcts, and 50% cases had delayed myelination on MRI.
In the current study, among the 18 (36%) patients evaluated with known history of preterm delivery, 11 (61.1%) had cerebral cortical atrophy, 10 (55.56%) had periventricular leukomalacia (PVL) as the most common findings, 9 (50%) had corpus callosum thinning, 8 (44.45%) had delayed myelination, 3 (16.67%) had germinal matrix hemorrhage, and 3 (16.67%) were diagnosed with acute infarcts. Serdaroglu et al16 evaluated 89 children with periventricular leucomalacia for neurodevelopmental delay. On MRI, periventricular leucomalacia is broadly classified into 4 grades as follows: grade IV shows diffuse paucity of cerebral deep white matter with ventricular dilatation, grade III shows periventricular and deep white matter hyperintensities numbering >3 with irregular wavy margins of the ventricular wall, grade II shows periventricular and deep white matter hyperintensities numbering >3, and grade I shows unilateral or bilateral discrete periventricular hyperintensities numbering <3. In their study, most prevalent findings were thinned out corpus callosum which was observed in 73% cases, cerebral cortical atrophy in 47.2% cases, and delayed myelination in 14.3% cases. Also, 18% cases had normal MRI findings. In a similar study done by Logitharajah et al,17 the major sites of hypoxic–ischemic injury were basal ganglia noted in 75% cases, periventricular and deep white matter noted in 89% cases, cerebral cortex noted in 58% cases, and brain stem noted in 44% cases. Also, MRI findings were normal in 32% cases. In their study, injury to the brainstem and central gray matter nuclei was found in many preterm cases with HIE. Findings from the current study, correlate with both the above studies.
Among eight patients with cerebral palsy in the current study, three (37.5%) were preterm and five (62.5%) were full term. On MRI, periventricular leukomalacia was the prevalent finding in preterm patients, and periventricular and deep white matter hyperintensities on T2 and FLAIR MRI sequences were most prevalent in full-term patients. Overall, the most common findings on MRI in patients with cerebral palsy in the current study were cerebral cortical atrophy noted in 7 (87.5%), deep white matter hyperintensities on T2 and FLAIR sequences noted in 6 (75%), corpus callosum thinning noted in 5 (62.5%), delayed myelination noted in four (50%), and periventricular leukomalacia noted in two (25%). In a study done by Yin et al on MRI findings in 42 cases, 12 [28%] premature cases and 30 full-term [72%]) cases, the most prevalent finding was periventricular leukomalacia noted in 28 (66.6%) cases, followed by cerebral cortical atrophy encountered in 14 (33.3%) cases. MRI findings were normal in 14 (33.3%) preterm cases. Also, the other group studied by Yin et al14 had 30 full-term cases with clinical findings of HIE and a history of birth asphyxia. On MRI, widely prevalent findings were periventricular and deep white matter hyperintensities on T2 and FLAIR sequences noted in approximately 30% cases, succeeded by cerebral cortical atrophy noted in 20% cases and subcortical lesions in another 20% cases. Additional findings encountered were acute infarcts noted in 13% cases, delayed myelination noted in 7% cases, and thinned out corpus callosum noted in 6% cases. Truwit et al18 performed a study on 40 cases (11 premature and 29 full-term infants) with clinical findings of cerebral palsy. On MRI, while the most common finding encountered was paucity of white matter predominantly in the terminal zone of myelination, the peritrigonal white matter, 81% cases had findings of thinned out corpus callosum. In their study, reduced caliber of the brainstem was noted in 27% cases, and no abnormalities were encountered in basal ganglia and thalami. Also, the other group included 29 full-term infants with HIE. On MRI sequences, the most prevalent findings were loss of deep white matter noted in 66% cases, focal or diffuse thinning of the corpus callosum noted in 55% cases, irregular ventricular margins with ventricular dilatation and resultant cerebral atrophy noted in 41% cases, and cerebral cortical atrophy noted in 10% cases. While basal ganglia or thalamic involvement was demonstrated in 20% cases, delayed myelination was noted in 6% cases, and one case had findings of cystic encephalomalacia. Findings from the current study correlate with both the above studies.
Limitations of the Study
The findings of the current study are not without limitations. First, external validity may be limited in the current study due to its single-center setup. In addition, the study is retrospective in nature, and thus the role of MRI in prognostic follow-up of preterm and full-term infants with HIE could not be determined and correlated. To determine prognosis of HIE related brain damage on MRI, further large-scale prospective studies that yield more robust data need to be undertaken. A third limitation is relatively small number of patients.
Conclusion
MRI detects lesions of hypoxic–ischemic injury to the brain with high accuracy and is the primary imaging modality of choice in preterm and full-term patients with history of perinatal birth asphyxia, as it helps determine localization and characterization of the underlying disease pattern based on the duration and severity ischemic insult. MRI has a prognostic role in providing an early diagnosis of HIE lesions which is required for prompt management. In the current study, distinguishable pattern of MRI findings secondary to birth asphyxia and ischemic insult were elucidated in both preterm and full-term patients who are highly dependent on the level of brain maturity at the time of imaging. Regular MRI follow-up has a prognostic significance in HIE with accurate prediction of neurodevelopmental outcome on follow up studies.
Acknowledgment
The author wishes to thank Mrs. Mani Sabbavarapu for her assistance in proofreading and native English editing of the manuscript.
Conflict of Interest
None declared.
Funding None.
References
- Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol. 2009;36(4):835-858. , vii
- [Google Scholar]
- Frequency of hypoxic-ischemic encephalopathy among hospitalized neonates in West Iran. Iran J Pediatr. 2010;20(2):244-245.
- [Google Scholar]
- Magnetic resonance imaging spectrum of perinatal hypoxic-ischemic brain injury. Indian J Radiol Imaging. 2016;26(3):316-327.
- [Google Scholar]
- Short and long term prognosis in perinatal asphyxia: An update. World J Clin Pediatr. 2016;5(1):67-74.
- [Google Scholar]
- Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics. 2008;28(2):417-439. , quiz 617
- [Google Scholar]
- MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27(3):533-547.
- [Google Scholar]
- Early detection of global cerebral anoxia: improved accuracy by high-b-value diffusion-weighted imaging with long echo time. AJNR Am J Neuroradiol. 2005;26(6):1487-1497.
- [Google Scholar]
- Advances in myelin imaging with potential clinical application to pediatric imaging. Neurosurg Focus. 2013;34(4):E9.
- [Google Scholar]
- Magnetic resonance imaging findings of term and preterm hypoxic-ischemic encephalopathy: a review of relevant animal models and correlation to human imaging. Open Neuroimaging J. 2018;12:55-65.
- [Google Scholar]
- Neonatal diffusion tensor brain imaging predicts later motor outcome in preterm neonates with white matter abnormalities. Ital J Pediatr. 2016;42(1):104.
- [Google Scholar]
- MRI in white matter diseases – clinico-radiological correlation. Indian J Radiol Imaging. 2002;12(1):43-50.
- [Google Scholar]
- Hypoxic ischemic encephalopathy in neonates. J Ayub Med Coll Abbottabad. 2010;22(4):190-193.
- [Google Scholar]
- Involvement of the anterior lobe of the cerebellar vermis in perinatal profound hypoxia. AJNR Am J Neuroradiol. 2007;28(1):16-19.
- [Google Scholar]
- Magnetic resonance imaging findings in cerebral palsy. J Paediatr Child Health. 2000;36(2):139-144.
- [Google Scholar]
- Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed. 1996;75(3):F145-F151.
- [Google Scholar]
- Correlative value of magnetic resonance imaging for neurodevelopmental outcome in periventricular leukomalacia. Dev Med Child Neurol. 2004;46(11):733-739.
- [Google Scholar]
- Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res. 2009;66(2):222-229.
- [Google Scholar]
- Cerebral palsy: MR findings in 40 patients. AJNR Am J Neuroradiol. 1992;13(1):67-78.
- [Google Scholar]







