Translate this page into:
Long-term follow-up in high-grade meningioma and outcome analysis
*Corresponding author: Anant Mehrotra, Department of Neurosurgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. dranantmehrotra@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Singh R, Kanjilal S, Mehrotra A, Misra S, Tataskar P, Mishra D. Long-term follow-up in high-grade meningioma and outcome analysis. J Neurosci Rural Pract. 2024;15:270-7. doi: 10.25259/JNRP_573_2023
Abstract
Objectives:
The determinants of progression-free survival (PFS) and overall survival (OS) for higher-grade meningiomas have not been clearly established and to summarize the long-term clinical outcome for patients with grade 2 or 3 meningioma and assess the PFS and OS factors.
Materials and Methods:
The study included all individuals, who had undergone surgical removal of cerebral meningiomas between 2005 and 2020 and whose histological results suggested a World Health Organization (WHO) grade 2 or grade 3 diseases. Kaplan–Meier curves are plotted to examine tumor control and OS after the follow-up. The reverse Wald logistic regression and Mantel-Cox test were used in multivariate analysis for tumor recurrence and mortality.
Results:
There were 94 individuals enrolled with 82 having WHO grade 2 tumors and 12 having WHO grade 3 lesions. Gross total resection of the tumor was present in 73 patients (78%), and adjuvant radiotherapy (RT) was administered to 43 (45.7%) individuals. During the course of the study, 17 patients died. The WHO grade of the tumor, the extent of resection, and the absence of bone involvement were all independent predictors of better survival in a multivariate analysis. Furthermore, whereas adjuvant RT after surgery enhanced survival, it was not statistically significant (hazard ratios [95% confidence interval CI] = 1.91 [0.15–23.52] [P = 0.61]).
Conclusion:
The degree of tumor excision is the strongest predictor of PFS and OS. In the event of a recurrence, rather than opting for upfront radiation, a second surgery with the goal of maximum safe resection should be performed.
Keywords
High-grade meningioma
Grade of resection
Outcome analysis
INTRODUCTION
Meningioma have been classified into three grades, grade 1 which is the most frequently occurring subtype, other being grade 2 in 5–34% cases, and grade 3 in 1–3% cases.[1,2] As per the latest Central Brain Tumor Registry of the United States statistical report, the most common non-malignant histopathology was meningioma (39.7% of all tumors and 55.4% of all non-malignant tumors).[2] Meningioma diagnosis is based on history, physical examination, and radiological investigations and histopathological examination of biopsy tissue. Immunohistochemistry, molecular, and cytogenetic studies in meningioma further stratify the grades of meningiomas. The recent 2021 World Health Organization (WHO) classification emphasized that the categorization of meningiomas to grade 2 or grade 3 should not be confined to the histological findings, but the presence of specific biomarkers determines the prognosis in such cases.[1] The presence of specific biomarkers such as TERT promoter mutations and homozygous deletions in the cell cycle regulator genes CDKN2A and/or CDKN2B has been been found to be associated with recurrent meningiomas and results in poor prognosis.[3,4] The predictors of progression-free survival (PFS) and overall survival (OS) for higher grades meningiomas have not been well established as compared to its benign equivalents. The management of WHO grade 2 and 3 often requires supplementary treatment in the form of fractionated radiotherapy (RT), stereotactic radiosurgery (SRS), and chemotherapy.[5,6]
Objectives
This single-center retrospective study aims to evaluate the clinical and radiological features and to summarize the long-term clinical course for patients diagnosed with grade 2 or 3 meningioma. The secondary objective was to evaluate PFS and OS.
MATERIALS AND METHODS
Patient selection and study setting
All the patients with age >18 years, who had undergone surgical resection of cranial meningiomas between the period of 2005–2020 in our institute, were included in the study. The 795 cranial meningiomas were surgically removed during the study period, of which 678 patients had WHO grade 1 tumors and were excluded from the study. Of the remaining 117 patients, another 23 patients were excluded; 14 had their initial surgery at another facility and were referred to our facility for recurrence or regrowth of residual lesions; and 9 patients were lost to follow up. The medical data for 7 of the 9 patients, who were lost to follow up was insufficient to ascertain whether they had undergone adjuvant RT [Figure 1]. The medical records of these 94 patients including clinical history, neurological condition at admission, operation notes, and histopathological reportswere obtained from our online database, hospital information system (HIS), and from the clinical files and records. The study has been reviewed and approved by the Institutional Ethical Committee.
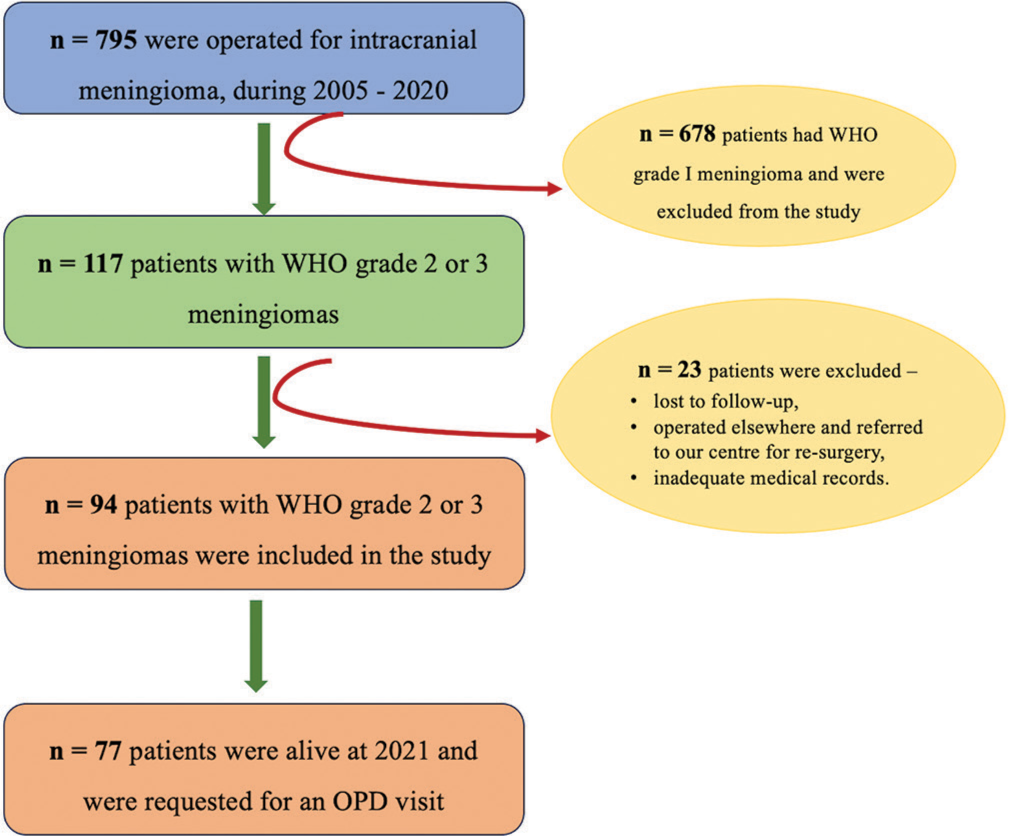
- Consort chart showing the patient inclusion process. n = Number of patients. WHO: World Health Organization. OPD: Outpatient department.
Exclusion criteria
The following criteria were excluded from the study:
Patients with histopathological diagnosis of WHO grade 1 meningioma (as we intend to determine the outcome in patients with high-grade meningiomas only).
Patients with inadequate medical documentation on the HIS.
Patients who have received adjuvant treatment at any other center.
Variables retrieved from the database
The demographic profile, presenting symptoms (headache, weakness, seizure, and cranial nerve deficit), and preoperative Karnofsky Performance Status (KPS), and presence of concomitant disorders were all documented. Tumor features such as tumor location, presence of peritumoral edema, and post-operative degree of resection were studied. The intensity of the tumor on T2-weighted MRI was assessed, and tumors that were isointense and hypointense were combined into one category whereas hyperintense lesions formed the other. The tumor consistency and vascularity were documented based the operating surgeons’ subjective evaluation. Tumor regrowth is defined as radiographic evidence of an increase in the size of the residual lesion by 15% each year in the follow-up MRI following subtotal excision[7] whereas tumor recurrence is defined as the demonstration of a new lesion following gross total excision. Various treatment modalities such as total numbers of surgeries and RT to which patients were subjected were also studied to assess the impact of these modalities. Considering the degree of resection, Simpson Grades I and II were regarded as gross total resection (GTR) for non-skull base meningiomas, and Simpson Grades III and IV were considered as subtotal resection (STR).[8] Similar to this for skull base meningioma, grade 1, grade 2, and grade 3 modified Okudera-Kobayashi resections are classified as GTR while grade 4 and grade 5 are classified as STR. The histopathological reports were reviewed and classified into WHO grade 1, 2 and 3 as per the WHO 2016 classification.
Follow-up
According to our department’s follow-up protocol, patients are asked to visit the outpatient department six weeks after surgery and again three months later with a standard contrast MRI. For patients with the WHO grade 1 lesion, repeat imaging is done at one year, three years, five years, and 10 years postoperatively. Annual MRI surveillance is performed on patients with grade 2 or grade 3 lesions. In this study, we have performed MR surveillance at three months, six months, and one year after surgery and once yearly thereafter. In the event of recurrence or regrowth of a residual lesion, a choice is reached through consultation with the neuroradiologist and radiotherapist, and the options are presented to the patients at the same time.
Statistical analysis
This was done in IBM Statistical Software for the Social Sciences Statistics Version 23.00 Armonk, New York, USA. The Shapiro– Wilks test was used to assess the normality of the data. The parametric continuous variables were expressed with mean ± standard deviation and for non-parametric data, median values (1st quartile–3rd quartile) while categorical variables were expressed with frequency and associated percentages. To ascertain any significant change in the KPS before and after surgery, the Wilcoxon matched-pairs signed-ranks test was used. Kaplan–Meier curves are plotted to look for tumor control and OS. Multivariate analyses for tumor recurrence and mortality were performed using the Mantel-Cox test and backward Wald logistic regression and characterized by hazard ratios (HR), 95% confidence intervals (CI), and the Wald test p values. Logistic regression models included patients’ age and sex (male = reference [ref]), tumor location (classified as non-skull base [ref] vs. skull base), extent of resection (EOR) (GTR [ref] vs. STR), pre-operative KPS, T2 intensity on MRI (classified as iso/hypointense [ref] vs. hyperintense), and adjuvant RT (yes = reference). The statistical significance was set to P < 0.05.
RESULTS
Clinical and radiographic characteristics
Of the 94 patients, 54 (57.5%) were men and 40 (42.5%) were women. The median age at diagnosis was 45 years (36–58 years). Eighty-two (87.2%) patients were diagnosed with WHO grade 2 meningiomas and 12 (12.8%) patients were WHO grade 3. The common presenting symptoms were headache with or without vomiting, seizures, and neurological deficit. The demographic and radiographic features of the patients are tabulated in Table 1. Convexity meningiomas were found in 31 (33%) of the cases followed by skull base meningiomas in 30 (31.9%) and parasagittal meningiomas in 17 (18.1%). On T2-weighted MRI, 23 (24.5%) of the tumors were hyperintense, 16 (17%) were hypointense, and 55 (58.5%) were isointense.
| Parameters | Total | Grade 2 n(%) | Grade 3 n(%) |
|---|---|---|---|
| Total no. of patients | 94 | 82 (87.2%) | 12 (12.8%) |
| Median age (1st Quartile – 3rd Quartile) |
45 years (36–58) |
45 years (34.75–58) |
50.5 years (37.25–60.25) |
| Gender Male Female |
54 (57.5%) | 45 (54.8%) | 9 (75%) |
| 40 (42.5%) | 37 (45.2%) | 3 (25%) | |
| Clinical symptoms Headache Neurological deficits Seizures |
68 (72.3%) | 60 (73.2%) | 8 (66.7%) |
| 56 (59.6%) | 51 (62.2%) | 5 (41.7%) | |
| 40 (42.6%) | 38 (46.3%) | 2 (16.7%) | |
| Location of tumour Convexity Parasagittal Falcine Tentorial Skullbase Intraventricular |
31 (33%) | 25 (30.5%) | 6 (50%) |
| 17 (18.1%) | 16 (19.5%) | 1 (8.3%) | |
| 9 (9.6%) | 8 (9.8%) | 1 (8.3%) | |
| 5 (5.3%) | 5 (6.1%) | 0 | |
| 30 (31.9%) | 26 (31.7%) | 4 (33.3%) | |
| 2 (2.2%) | 2 (2.4%) | 0 | |
| T2 weighted MRI intensity Isointense Hypointense Hyperintense |
55 (58.5%) | 51 (62.2%) | 4 (33.3%) |
| 16 (17%) | 16 (19.5%) | 0 | |
| 23 (24.5%) | 15 (18.3%) | 8 (66.7%) | |
| Median Pre-operative KPS (1st Quartile – 3rd Quartile) |
70 (60–70) |
70 (60–80) |
55 (42.5–60) |
| Median Post-operative KPS (1st Quartile – 3rd Quartile) |
70 (70–80) |
80 (70–80) |
30 (30–40) |
| Extent of resection GTR (Simpson Grade I and II) STR (Simpson Grade III and IV) |
73 (77.7%) | 69 (84.1%) | 4 (33.3%) |
| 21 (22.3) | 13 (15.9%) | 8 (66.7%) | |
| Radiotherapy | 43 (45.7%) | 41 (50%) | 2 (16.7%) |
| Recurrences | 40 (42.6%) | 29 (35.4%) | 11 (91.7%) |
| Mortality | 17 (18.1%) | 8 (9.8%) | 9 (75%) |
n: Number of patients. GTR: Gross total resection, STR: Subtotal resection, KPS: Karnofsky Performance Status
Surgical resection
The 92 of the 94 patients had open surgery while the remaining two got endoscopic surgery. The consistency of the tumor as reported by the operating surgeon was found to be firm in 63.8% of the instances, soft in 34% of the cases, and hard in just 2.1% of the cases. In 32% of cases, the tumor was very vascular while 46.8% and 21.3% of the tumors exhibited moderate and low vascularity, respectively. Gross total excision was performed in 73 (77.7%) patients while 21 (22.3%) received subtotal excision of the tumor with 19 individuals having encasement of the neighboring neurovascular systems. The 19 of the 25 patients with implicated bones had meningiomas in their skull bases making it impossible to remove the affected bone and attach mesh.
Post-operative course
In the post-operative period, 75 (79.7%) patients reported relief in their symptoms. During the same hospital stay, 9 (10%) patients underwent re-surgery. The most common reason for re-exploration was a developing infarct in five patients, hydrocephalus requiring ventriculoperitoneal shunt insertion in two patients, and surgical site hematoma evacuation in the other two patients.
RT
During the treatment course, 41 (43.6%) patients received supplementary fractionated RT with a cumulative dose 50–60 Gy.
Outcomes
The median duration of follow-up was 10.19 years. During this time, 17 (18%) patients died with 8 (9.8%) having WHO grade 2 tumors and 9 (75%) having WHO grade 3 tumors (P < 0.001). The median pre-operative KPS was 70 (Q1–Q3: 60–70) (cares for self, unable to carry out normal activities or work). Surgery was associated with statistically significant improvement in the KPS postoperatively (median = 70 [Q1–Q3: 70–80]) (P = 0.048) [Figure 2].
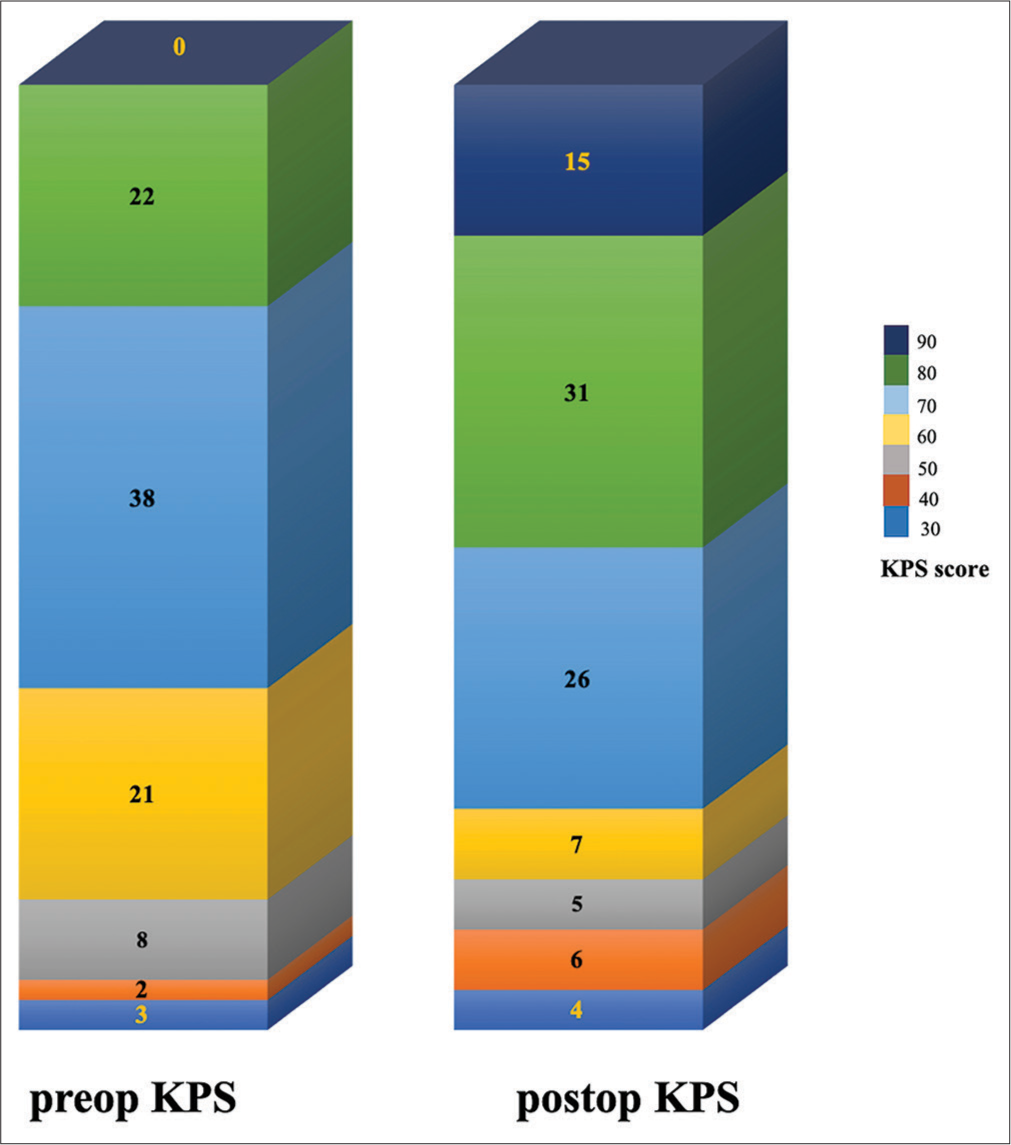
- A bar graph displaying the karnofsky performance status (KPS) before and three to six months after surgery (The numbers within the graph represents the total no. of patients in that category). Preop: Preoperative, Postop: Postoperative.
PFS
Recurrence or regrowth of residual lesion was observed in 41 (43.6%) of the patients, 29 (35.4%) patients with WHO grade 2 tumors, and all (n = 12) patients with the WHO grade 3 tumors (P < 0.001). The PFS for these high-grade meningiomas were 95% at the end of one year, 52% at five years, and 27.5% at 10 years. The PFS with the WHO grade 2 lesion was 8.43 years while it was 2.45 years with the WHO grade 3 lesion [Figure 3a] and was statistically significant (P < 0.001). Similarly, those who had GTR had a PFS of 8.65 years compared to 3.46 years for those who had STR (P < 0.001) [Figure 3b]. Patients who got irradiation after surgery had a PFS of 8.65 years compared to 3.34 years for those who did not receive radiation and was statistically significant (P = 0.039) [Figure 3c]. The intensity of the lesion on T2-weighted MRI also had an effect on PFS, which was 8.35 years for an iso/hypointense lesion, 1.84 years for a hyperintense lesion, and was statistically significant (P < 0.001) [Figure 3d].
![Selected Kaplan–Meier plots of progression-free survival (PFS) for high-grade meningiomas. In cumulative analyses, (a) median PFS was 8.43 years (95% confidence interval [CI] = 7.0–9.85 years) with grade 2 meningiomas and 2.45 years (95% CI = 1.69–3.2 years) with grade 3 meningiomas, (b) the median PFS was 8.65 years (95% CI = 5.38–11.9 years) after gross total resection and 3.46 years (95% CI = 2.58–4.33 years) after subtotal resection, (c) the median PFS after surgery and adjuvant irradiation was longer, 8.65 years (95% CI = 5.8–11.5 years) than after surgery alone 3.34 years (95% CI = 2.27–4.4 years), and (d) the median PFS for iso/hypointense lesion was 8.35 years (95% CI = 4.21–12.48 years) while that of hyperintense lesion was 1.84 years (95% CI = 0.83–2.84 years). WHO: World Health Organization, GTR: Gross total resection, STR: Subtotal resection.](/content/150/2024/15/2/img/JNRP-15-270-g003.png)
- Selected Kaplan–Meier plots of progression-free survival (PFS) for high-grade meningiomas. In cumulative analyses, (a) median PFS was 8.43 years (95% confidence interval [CI] = 7.0–9.85 years) with grade 2 meningiomas and 2.45 years (95% CI = 1.69–3.2 years) with grade 3 meningiomas, (b) the median PFS was 8.65 years (95% CI = 5.38–11.9 years) after gross total resection and 3.46 years (95% CI = 2.58–4.33 years) after subtotal resection, (c) the median PFS after surgery and adjuvant irradiation was longer, 8.65 years (95% CI = 5.8–11.5 years) than after surgery alone 3.34 years (95% CI = 2.27–4.4 years), and (d) the median PFS for iso/hypointense lesion was 8.35 years (95% CI = 4.21–12.48 years) while that of hyperintense lesion was 1.84 years (95% CI = 0.83–2.84 years). WHO: World Health Organization, GTR: Gross total resection, STR: Subtotal resection.
OS
The OS rate at 5 years, 10 years, and 15 years were 91.3%, 81.9%, and 78.4%, respectively. Patients with grade 2 lesions had an median survival of 16.7 years whereas patients with WHO grade 3 lesions had an average survival of 5.22 years (P < 0.001). Median survival for patients who had GTR was 16.99 years while it was 10.66 years for those who had STR of the tumor. Postoperative irradiation increases OS from 14.14 years in non-radiated patients to 16.85 years in irradiated patients [Figure 4].
![Selected Kaplan–Meier plots of progression-free survival for high-grade meningiomas. In cumulative analyses, (a) median survival was 16.7 years (95% confidence interval [CI] = 15.85–17.69 years) with grade 2 meningiomas while only 5.22 years (95% CI = 4.04–6.41 years) with grade 3 meningiomas, (b) the median survival was 16.99 years (95% CI = 16.09–17.89 years) after gross total resection and 10.66 years (95% CI = 7.6–13.72 years) after subtotal resection’, (c) the median survival after surgery and adjuvant irradiation was longer, 16.85 years (95% CI = 15.61–18.09 years) than after surgery alone 14.14 years (95% CI = 12.3–15.98 years), and (d) the median survival for iso/hypointense lesion was 16.73 years (95% CI = 15.75–17.72 years) while that of hyperintense lesion was 11.45 years (95% CI = 8.41–14.49 years). WHO: World Health Organization, GTR: Gross total resection, STR: Subtotal resection](/content/150/2024/15/2/img/JNRP-15-270-g004.png)
- Selected Kaplan–Meier plots of progression-free survival for high-grade meningiomas. In cumulative analyses, (a) median survival was 16.7 years (95% confidence interval [CI] = 15.85–17.69 years) with grade 2 meningiomas while only 5.22 years (95% CI = 4.04–6.41 years) with grade 3 meningiomas, (b) the median survival was 16.99 years (95% CI = 16.09–17.89 years) after gross total resection and 10.66 years (95% CI = 7.6–13.72 years) after subtotal resection’, (c) the median survival after surgery and adjuvant irradiation was longer, 16.85 years (95% CI = 15.61–18.09 years) than after surgery alone 14.14 years (95% CI = 12.3–15.98 years), and (d) the median survival for iso/hypointense lesion was 16.73 years (95% CI = 15.75–17.72 years) while that of hyperintense lesion was 11.45 years (95% CI = 8.41–14.49 years). WHO: World Health Organization, GTR: Gross total resection, STR: Subtotal resection
Regression analysis
Univariate analysis
The PFS is correlated with the degree of resection and WHO grade of the lesion. Comparing those who underwent STR to those who received GTR, those who underwent STR had a 53-fold higher risk of recurrence. As opposed to this, the risk of recurrence with a WHO grade 3 lesion is 20 times higher than one with a WHO grade 2 lesion. Improved OS was associated with the T2 intensity on MRI, degree of resection, involvement of the bone, WHO grade, and postoperative irradiation. The analysis’ findings are summarized in Table 2.
| Parameters | Progression free survival | Overall survival | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | P-value | Hazard Ratio (95%CI) | P-value | |
| Age | 0.98 (0.96–1.0) | 0.41 | 0.99 (0.96–1.035) | 0.94 |
| Gender | 1.6 (0.70–3.66) | 0.25 | 0.35 (0.11–1.14) | 0.084 |
| Location* | 1.28 (0.53–3.06) | 0.58 | 0.65 (0.21–2.04) | 0.466 |
| T2 intensity** | 1.67 (0.65–4.31) | 0.28 | 0.09 (0.029–0.33) | <0.001a |
| Preop KPS$ | 0.42 (0.18–1.00) | 0.51 | 10.36 (2.66–40.27) | 0.001 |
| Extent of excision | 53.0 (6.67–421.3) | <0.001a | 18.97 ( 5.05–71.22) | <0.001a |
| Bone involvement | 0.74 (0.29–1.85) | 0.52 | 4.16 (1.3–13.13) | 0.015a |
| WHO grade | 20.1 (2.47–163.6) | 0.005a | 38.0 (8.07–178.78) | <0.001a |
| Radiotherapy | 1.13 (0.49–2.56) | 0.76 | 4.1 (1.07–15.66) | 0.039 a |
Multivariate analysis
The EOR and WHO grade of tumor linked with tumor recurrence in a multivariate analysis. In compared to patients who had STR, patients who underwent GTR exhibited a 17-fold increase in the likelihood of PFS. Similarly, individuals with the WHO grade 3 lesions had a tenfold increased risk of tumor recurrence. The only predictors of mortality were EOR, bone involvement, and WHO grade of the tumor. The results of the analyses are summarized in Table 3.
| Parameters | Progression free survival | Overall survival | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | P-value | Hazard Ratio (95%CI) | P-value | |
| Age | 0.97 (0.94–1.0) | 0.105 | 0.98 (0.91–1.06) | 0.66 |
| Gender | 2.25 (0.77–6.58) | 0.136 | 13.19 (0.94–173.2) | 0.07 |
| Location* | 0.71 (0.22–2.24) | 0.56 | 0.25 (0.03–1.82) | 0.17 |
| T2 intensity** | 0.31 (0.66–1.45) | 0.13 | 2.79 (0.37–20.93) | 0.31 |
| Preop KPS$ | 0.98 (0.93–1.03) | 0.56 | 0.23 (0.37–1.45) | 0.12 |
| Extent of excision | 17.01 (3.49–82.73) | <0.001a | 23.61 ( 3.45–161.28) | 0.001a |
| Bone involvement | 1.41 (0.4–4.95) | 0.58 | 6.9 (1.0–47.56) | 0.05a |
| WHO grade | 10.27 (1.09–96.52) | 0.042a | 23.0 (3.21–164.69) | 0.002a |
| Radiotherapy | 0.54 (0.17–1.69) | 0.29 | 1.91 (0.15–23.52) | 0.61 |
DISCUSSION
The majority of these tumors are WHO grade 1 with a low risk of recurrence and less aggressive behavior. The exceedingly low occurrence of high-grade meningiomas in the general population makes it difficult to predict the clinical prognosis. Our study’s GTR rate was 77.7%, which is consistent with the greatest series in the literature, which revealed a range of 48–87.3%.[9-11] In another study, it was found that the PFS at five years was 78.8% following GTR and 69.7% for those who underwent STR.[12] Although, the five-year PFS was 75% following GTR in our series, it was only 30% following STR. In fact, patients in our research, who underwent STR, had a 17-fold greater risk of recurrence.
The literature on the impact of GTR on the OS is contentious. The five-year survival rate for GTR was 98% whereas it only 66% for those who has undergone STR, a finding similar to the study conducted by Simonetti et al. where they have found the five-year survival to be 95% and 67% for patients with GTR and STR, respectively.[13] In our study, individuals who received GTR of the tumor had a 23-fold improved likelihood of survival. As a result, it backs up the current recommendation for maximum safe excision in high-grade meningiomas.[5] In addition, the subset of patients, who had STR demonstrated a gradual decline in PFS after one year following surgery as shown in [Figure 3b], and this group of patients requires vigilant monitoring to ensure early clinical or radiological evidence of recurrence and prompt initiation of appropriate therapy.
In our series, 43 (45.7%) patients, who received adjuvant RT, had a four-fold increased likelihood of survival on univariate analysis, but failed to show any positive correlation on multivariate analysis. The five-year PFS in those who have undergone STR was 74% in patient who received adjuvant RT versus 33% in those who have not been irradiated postoperatively. Similar findings indicated that while radical surgery with adjuvant RT is advised for the WHO grade 3 lesions, its usefulness in patients with WHO grade 2 lesions who had GTR is dubious.[14] Therefore, we suggest that all patients with grade 3 meningioma should be subjected to adjuvant RT while for patients with WHO grade 2, RT should be offered to only those patients in whom GTR could not be achieved. In the event of tumor recurrence or regrowth of residual lesions, they should be planned for re-surgery with the aim at attaining maximal safe resection, which provided that the patient’s general condition is amenable to surgery.
Stereotactic RT may be delivered as a single high dose (SRS) or as many as five fractions. The SRS is a valuable alternative available in a neurosurgeon’s armamentarium, especially for smaller tumors and tumors residing at an acceptable distance from brainstem or optic chiasma. In a study, the five-year PFS ranged from 25% to 83% for grade 2 meningiomas while it was 0–72% for grade 3 meningiomas.[15] The fractionated regimen offered better 10-year local control 91% in contrast to 74% with single-dose regimen.[16]
The prognostic significance of tumor site in high-grade meningioma and its impact on OS is debated and briefly discussed in the present literature. We classified tumors into skull base and non-skull base tumors in our study and observed no link between tumor site and OS or PFS, which is consistent with earlier research.[17,18] The EOR is the sole measure with reliable predictive capacity as evidenced by the high correlation between the EOR and the PFS and OS and the loss of relevance of tumor site in the multivariate model in our study. Furthermore, due to the limited surgical access and close proximity to neurovascular pathways, it is frequently difficult to achieve GTR of tumors that involve the skull base.[19] This explains the increase risk of recurrence of tumors in these locations and supplementation with adjuvant RT even in patients with the WHO grade 1 meningioma.[20]
The histopathological subtypes of meningioma include the commonly occurring meningothelial, fibroblastic, and transitional variety. While the less frequently encountered subtypes include metaplastic, lipomatous, osseous, cartilaginous, myxoid, xanthomatous, psammomatous, secretory, and angiomatous.[21] Histopathological findings have a well-established prognostic value with poor prognosis associated with the WHO grade 3 meningiomas.[22] The reported OS in a series at 5 years and 10 years for grade 3 meningioma was 44% and 14.2% while for grade 2 meningioma was 78.4% and 53.3%, respectively.[18] Our results are generally consistent with these data; the OS at 5 years and 10 years for grade 3 lesions is 41.7% and 15.6%, respectively while for grade 2 meningiomas, it is 94.8% and 87.8%.
CONCLUSION
This study emphasizes that in individuals with high-grade meningiomas, the extent of excision of the tumor is the primary indicator of favorable prognosis, both in terms of PFS and OS. In case of recurrence or regrowth of residual lesion, a second operation should be performed with the goal of achieving the maximum safe resection, either alone or in combination with adjuvant radiation. Adjuvant radiation should be added for patients with skull base meningiomas that have bone involvement, regardless of the visual indication of complete resection and those with STR of tumor, as it was found to prolong the PFS. However, the use of adjuvant RT as a routine course of treatment for all patients is discouraged because it does not improve survival rates.
The histopathological grade, scope of the resection, and bone involvement were all found to be independent predictors of survival by the multivariate analysis.
Ethical approval
The study was reviewed and approved by the Institutional Ethics Committee.
Declaration of patient consent
Patient’s consent is not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23:1231-51.
- [CrossRef] [PubMed] [Google Scholar]
- CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021;23:i1-105.
- [CrossRef] [PubMed] [Google Scholar]
- TERT promoter mutations are associated with poor prognosis and cell immortalization in meningioma. Neuro Oncol. 2018;20:1584-93.
- [CrossRef] [PubMed] [Google Scholar]
- CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140:409-13.
- [CrossRef] [PubMed] [Google Scholar]
- EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383-91.
- [CrossRef] [PubMed] [Google Scholar]
- Volumetric analysis of the growth rate of incompletely resected intracranial meningiomas. Zentralbl Neurochir. 2005;66:17-23.
- [CrossRef] [PubMed] [Google Scholar]
- The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
- [CrossRef] [PubMed] [Google Scholar]
- Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer. 2015;121:4376-81.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical meningiomas: Recurrence, reoperation, and radiotherapy. World Neurosurg. 2015;84:839-45.
- [CrossRef] [PubMed] [Google Scholar]
- The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013;115:241-7.
- [CrossRef] [PubMed] [Google Scholar]
- Conditional recurrence-free survival after surgical resection of meningioma. Neurosurgery. 2023;93:339-47.
- [CrossRef] [PubMed] [Google Scholar]
- Long term follow up in 183 high grade meningioma: A single institutional experience. Clin Neurol Neurosurg. 2021;207:106808.
- [CrossRef] [PubMed] [Google Scholar]
- EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021;23:1821-34.
- [CrossRef] [PubMed] [Google Scholar]
- The role of radiosurgery in the management of WHO Grade II and III intracranial meningiomas. Neurosurg Focus. 2013;35:E16.
- [CrossRef] [PubMed] [Google Scholar]
- Hypofractionated stereotactic radiotherapy for intracranial meningioma: A systematic review. Neurooncol Pract. 2019;6:346-53.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of intracranial meningioma. Neurosurgery. 2005;57:1088-95.
- [CrossRef] [PubMed] [Google Scholar]
- WHO grade II and III meningiomas: A study of prognostic factors. J Neurooncol. 2009;95:367-75.
- [CrossRef] [PubMed] [Google Scholar]
- Extent of resection in meningioma: Predictive factors and clinical implications. Sci Rep. 2019;9:5944.
- [CrossRef] [PubMed] [Google Scholar]
- Skull base meningiomas: Is surgical resection enough? Outcome evaluation and prognostic factors analysis in a single-center cohort. J Neurol Surg A Cent Eur Neurosurg. 2022;83:516-22. Erratum in: J Neurol Surg A Cent Eur Neurosurg 2022,83:e1
- [CrossRef] [PubMed] [Google Scholar]
- Transitional meningioma benign who grade 1 tumor-A case and review of the literature. IP Arch Cytol Histopathol Res. 2023;2023:20249.
- [Google Scholar]
- Meningiomas In: WHO classification tumours central nervous system. Lyon: International Agency for Research on Cancer IARC; 2007.
- [Google Scholar]






