Translate this page into:
Immunohistochemical expression of glial fibrillary acidic protein and CAM5.2 in glial tumors and their role in differentiating glial tumors from metastatic tumors of central nervous system
Address for correspondence: Dr. Ashima Batra, Department of Pathology, Pandit B. D. Sharma PGIMS, Rohtak - 124 001, Haryana, India. E-mail: drashimabatra@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objectives:
Immunohistochemistry (IHC) has become an important tool in the diagnosis of metastatic brain tumors. The judicious use of a panel of selected immunostains is unquestionably helpful in diagnostically challenging cases. In our study, the best combination of immune markers useful in differentiating metastatic carcinoma from high-grade gliomas in central nervous system (CNS) are glial fibrillary acidic protein (GFAP) and cytokeratin (CK) (CAM5.2).
Materials and Methods:
The study was conducted on 80 cases of glial tumors including metastatic tumors to the CNS. Histopathological diagnosis was established on routine hematoxylin and eosin staining of the sections. Special IHC markers, GFAP, and CAM5.2 were used to differentiate glial from metastatic tumors.
Result:
Of total 80 cases, 40 cases of astrocytic tumors, 2 cases of ependymoma, 2 cases of mixed glial tumors, and 16 cases of glioblastoma multiforme were positive for GFAP. Twelve cases of oligodendroglioma were negative for GFAP. The sensitivity of GFAP in glial tumors was statistically significant as 81.1% and specificity 100%, whereas sensitivity and specificity of CAM5.2 in metastatic tumors were 100%.
Conclusion:
IHC plays an important role in diagnosing tumors of CNS and markers such as GFAP and CK (CAM5.2) are quite effective in differentiating glial tumors from metastatic tumors of CNS.
Keywords
Glial fibrillary acidic protein
glial tumors
glioblastoma multiforme
metastatic tumors
CAM5.2
Introduction
The subject of tumors of the nervous system is often looked upon with apprehension by medical students and practitioners, with some justification. The plethora of terminologies and systems of grading and classification, the bewildering numbers of separately named lesions, and their apparently endless histologic variations provide ample basis for their perception. Central nervous system (CNS) tumors are the neoplasms constituting 1-2% of all the neoplasms. Astrocytomas are the most common primary tumors. Gliomas constitute 38.7% of CNS tumors in which high-grade gliomas are 59.5% and low-grade gliomas are 33.1%.[1] CNS is also the most common target of metastatic dissemination. Ten to 50% of patients with systemic malignancies develop brain metastasis during their disease.[2] The incidence of metastasis from lung carcinoma is 18-60%, breast carcinoma 5-21%, melanoma 4-16%, genitourinary 310%, while gastrointestinal malignancies constitute 5-12%. Immunohistochemistry (IHC) has become an important tool in the diagnosis of brain tumors. Although conventional hematoxylin and eosin (H and E) staining is the mainstay for pathologic diagnosis, IHC has played a major role in differential diagnosis and in improving diagnostic accuracy not only in general surgical pathology but also in neuro-oncologic pathology. The judicious use of a panel of selected immunostains is unquestionably helpful in diagnostically challenging cases. In addition, IHC is also of great help in predicting the prognosis for certain brain tumors.[3]
IHC using monoclonal or polyclonal antibodies has greatly influenced the diagnosis of various neurological disorders. Using this technique, the presence of characteristic antigen can be precisely defined in a sensitive and reproducible manner, thereby providing a better tool for making an accurate diagnosis of brain tumors.[4]
Glial fibrillary acidic protein (GFAP) was first isolated from old multiple sclerosis plaques by Roy and Sarkar. It has a molecular weight ranging from 40 to 50 KD. It is normally present in the astrocytes (mature and developing), ependymal cells, and radial glia of developing the brain. GFAP is the most frequently used marker in diagnostic neuro-oncology. Positive reaction to GFAP has been demonstrated in astrocytomas, ependymoma, and astrocytic cells of mixed gliomas, subependymal giant cell astrocytoma, pleomorphic xanthoastrocytoma, astroblastoma, and gliosarcoma.[5]
Cytokeratins (CKs) monoclonal antibodies are useful in identification of the epithelial nature of the neoplasm. These antibodies are most useful in the differentiation of poorly differentiated epithelial malignancies from those other nonepithelial neoplasm. Group A (acidic) and Group B (basic) keratins are separated into 19 members by molecular weight, charge specificities, and immunoreactivity with monoclonal antibodies with restricted specificities. CAM5.2 is the mouse monoclonal antibody raised against colon carcinoma cell line HT29. It has been shown to react with one Group A keratin (molecular weight 50 KD) and two Group B keratins (molecular weight 43 KD and 39 KD). It is a low molecular weight CK. It stains normal epithelial cell with the exception of stratified squamous epithelium.[6]
High-grade gliomas are difficult to differentiate from metastatic tumors on the basis of light microscopy alone. Various studies have been conducted on metastatic brain tumors using nonspecific CKs. The role of CAM5.2 being highly specific is emerging as a specific marker to diagnose metastatic carcinoma. Hence, this study is planned to differentiate glial tumors from metastatic tumors with the help of IHC (CAM5.2 and GFAP).
Materials and Methods
The present study was conducted over a period of 2 years, from December 2010 to December 2012 in Department of Pathology, Pandit B.D. Sharma PGIMS, Rohtak and comprised of total 80 cases of CNS tumors including metastatic tumors to the CNS. The pattern of expression of GFAP and CAM5.2 was studied in these cases. Histopathological diagnosis was established on routine H and E staining of the sections. Special IHC markers (GFAP and CAM5.2) were used to differentiate high-grade gliomas from metastatic tumors. Sections of the human brain were run with each batch of IHC stains to act as a positive control for GFAP and skin biopsy as a positive control for CAM5.2. A negative control was obtained by substituting the primary antibody with an antibody of unrelated specificity.
Observation and Results
Over a period of 2 years, we examined total 80 cases of CNS tumors including metastatic tumors to the CNS [Figure 1]. In our study, the average age for the glial tumor was 35 years and for the metastatic tumor was 55 years. Among glial tumors, 71% were male, and 29% were female in a ratio of 2.5:1, whereas in metastatic tumors male to female ratio was 2:1 showing male preponderance. Radiological findings based on contrast-enhanced computer tomography (CT) revealed that the maximum cases of glial tumors were nonenhancing as compared to metastasis, which were enhancing and hypodense.

- Frequency of various histologic subtypes of glial and metastatic tumors
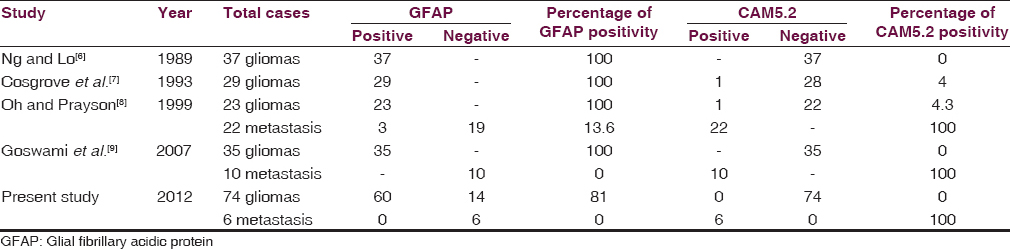
In the present study, glial tumors were widely reactive for GFAP [Figures 2 and 3a–b] except few oligodendrogliomas [Figure 3c and d] and mixed glial tumors and metastatic tumors were negative for GFAP and reactive for CAM5.2 [Figures 4–6]. Glial tumors, however, did not express CAM5.2. GFAP expression in glial tumors was statistically significant (sensitivity 81.1% and specificity 100%) as compared to metastatic tumors. CAM5.2 expression in metastatic tumors was also statistically significant (sensitivity 100% and 100% specificity) [Table 1].
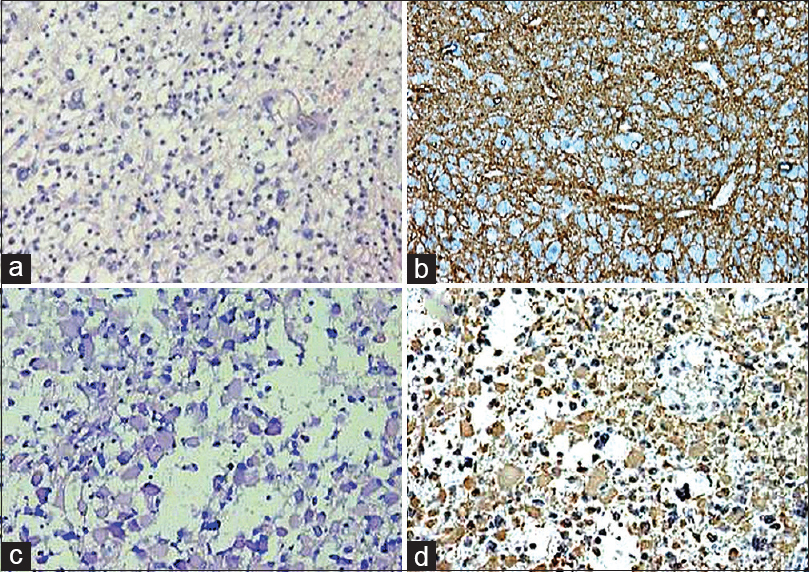
- Grade I astrocytoma (a; H and E, ×200) and gemistiocytic astrocytoma (c; H and E, ×200). Glial fibrillary acidic protein was positive in both the tumors (b; immunohistochemistry, ×100) (d; immunohistochemistry, ×200)
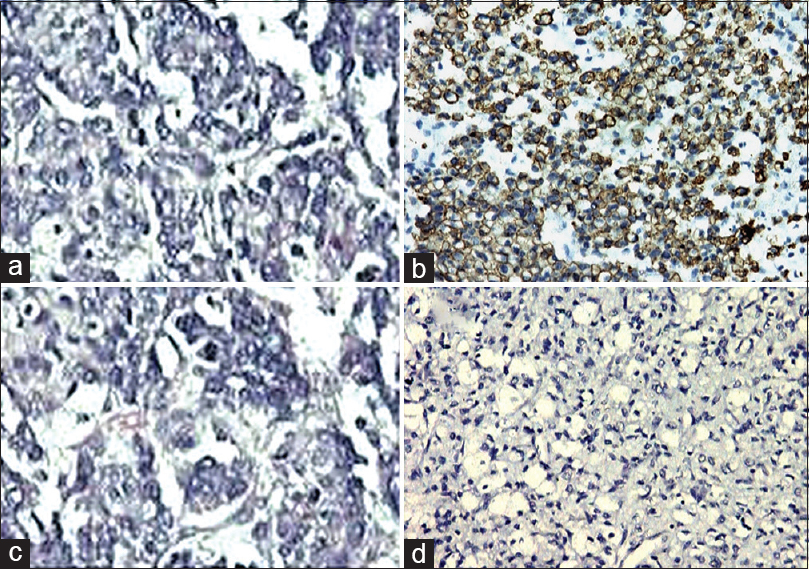
- Glioblastoma multiforme (a; H and E, ×40) revealing glial fibrillary acidic protein positivity (b; immunohistochemistry, ×100). However, oligodendroglioma (c; H and E, ×100) did not show positivity for glial fibrillary acidic protein (d; immunohistochemistry, ×400)
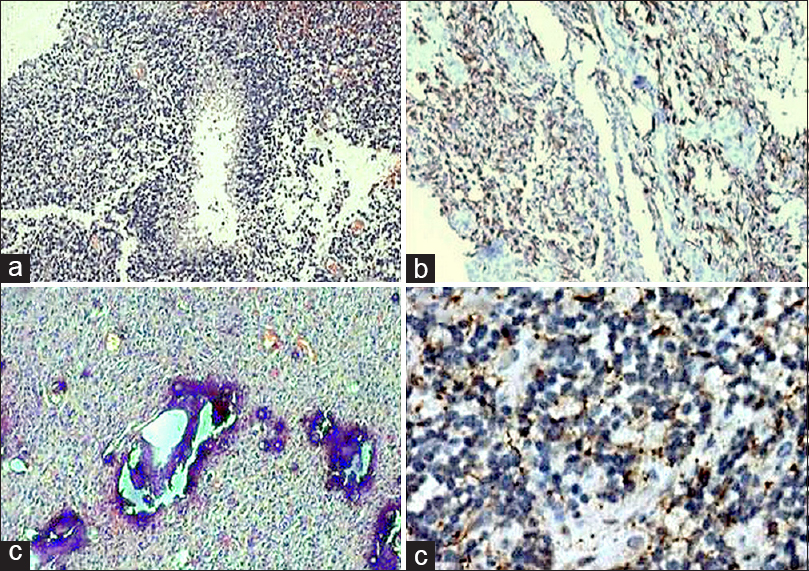
- Metastatic adenocarcinoma (a; H and E, ×100) revealing CAM5.2 positivity (b; immunohistochemistry, ×200)
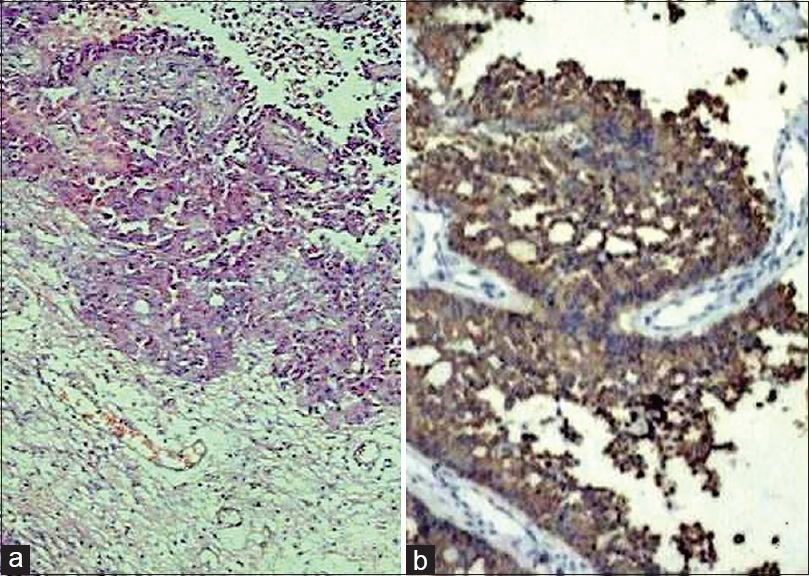
- Metastatic renal carcinoma (a; H and E, ×200) revealing CAM5.2 positivity (b; immunohistochemistry, ×200) and glial fibrillary acidic protein negativity (c; immunohistochemistry, ×200)
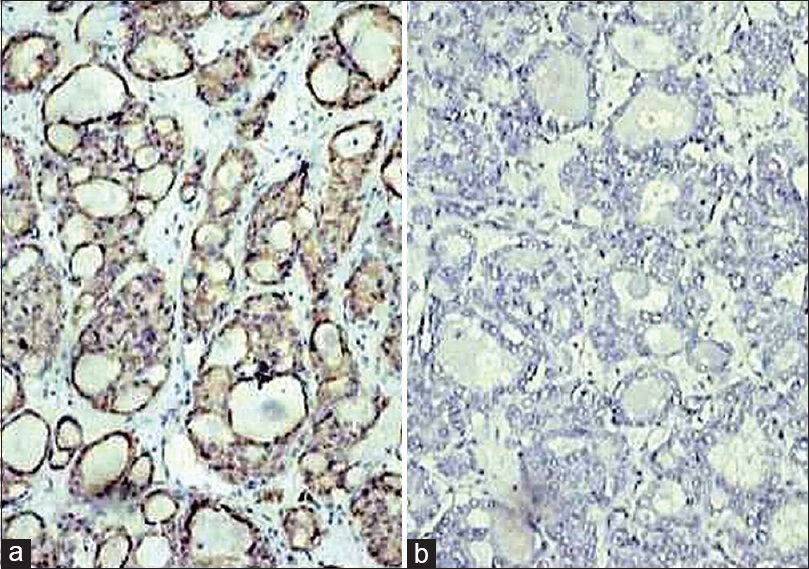
- Photomicrograph showing CAM5.2 positivity (a;IHC;×200) and GFAP negativity (b;IHC;×200) in metastatic follicular carcinoma thyroid
Discussion
Conventional H and E staining is crucial for diagnostic neuropathology. However, a number of markers for IHC have been developed. Some of the IHC markers are relatively sensitive and specific for some tumors (e.g., GFAP for astrocytomas). GFAP is currently used as a routine antigenic marker for normal developing and mature astroglial cells and for pathologically altered astrocytes. This protein is typically absent in primitive or neoplastic neuroepithelial cells, ganglion cells, oligodendrocytes, vascular endothelium, meningeal cells, fibroblasts, and other mesenchymal elements. The value of immunoperoxidase stain for GFAP is relevant in documenting astrocytic differentiation in the tumor growing outside the CNS parenchyma. The false negative reaction may occur as a consequence of delayed or improper fixation, presumably because much of the GFAP is in the soluble form and that can leach from the tissue before it is processed appropriately.[10]
GFAP found to be highly specific and sensitive marker when compared with other special stains and markers. It was found of particular importance in demonstration of astrocytic cellular differentiation in primitive or highly anaplastic CNS tumors, in study of mixed glial tumors, demonstration of glial nature of tumors, and diagnosing glial tumors invading meninges and extraneural sites.[11]
Glial filament immunoreactivity had been observed in tumor cells of glial origin and in tumor cells with foci of glial differentiation arising within CNS. No glial immunoreactivity was observed outside the CNS. The value of the immunoperoxidase stain for GFAP is particularly relevant in documenting astrocytic differentiation in tumors growing outside the CNS. It is, therefore, required for each center to determine the sensitivity and specificity of GFAP staining under their own conditions.[12]
CAM5.2 is an IgG2a murine monoclonal immunoglobulin composed of lower molecular weight CK proteins (50,000, 43,000, and 39,000 daltons). The CK identified by CAM5.2 corresponds to 8, 18, and 19. Comparison of CAM5.2 with other epithelial markers showed that it was most effective in discriminating between benign and malignant lesions.[13]
Some of nonspecific CK stain glial tumors strongly. It is critical to keep this point in mind when faced with the differential diagnosis of high-grade gliomas versus metastatic tumors in brain biopsies. It would be appropriate to go with highly specific low molecular weight CK (CAM5.2), which are positive in metastatic tumors and do not stain glial tumors.[14]
The wide variation in different series as shown in Table 1 may be due to various factors such as difference in source of material that is surgical or autopsy, the type of the concerned hospital that is neurological or surgical and the hospital concerned may serve a selective population of older patients. It also depends on the tendency of a neurosurgeon to avoid a biopsy if a primary site is known and multiple lesions are seen in the brain in CT scan.
High-grade astrocytic malignant neoplasms are difficult to distinguish from poorly differentiated metastatic carcinomas, particularly on small open biopsies. Brain biopsies performed specifically for diagnostic purpose rather than for tumor removal are small. Metastatic tumors seen in CNS may occasionally be difficult to distinguish histologically from high-grade gliomas. Glioblastoma multiforme (GBM) is characterized histologically by the presence of nuclear atypia, high mitotic rate and presence of necrosis, all characteristics often encountered in poorly differentiated metastatic carcinoma. The absence of well differentiated foci of metastatic carcinoma may make a diagnosis of metastatic carcinoma versus GBM difficult by light microscopic examination alone. Early detection of cancer and effective treatment increases the longevity of cancer patients.
In the present study, astrocytomas, as expected, were uniformly positive for GFAP. The staining pattern was more like similar among them. The staining pattern of GFAP in anaplastic astrocytomas was more variable. More anaplastic foci showed less positivity. Besides helping in the origin of histopathologically difficult cases of anaplastic astrocytoma, GFAP immunostain helped in the objective assessment of the degree of differentiation in these neoplasms. Astrocytic nature of the tumor cells was confirmed by demonstration of GFAP in less anaplastic areas. Positive GFAP immunostaining helped to overcome histopathological diagnostic problems in some undifferentiated tumors and to categorize these tumors as GBM. Use of GFAP and CAM5.2 immunomarker easily distinguished these two lesions in the occasional cases where light microscopic evaluation proven insufficient.
GFAP is a superior marker to S-100 and vimentin as observed by few authors based on immunostaining pattern of GFAP, vimentin, and S-100 using IHC techniques in human glial tumors.[15]
Perry et al. studied the diagnosis of metastatic adenocarcinomas to the brain of unknown primary. Sixty-eight cases of metastatic adenocarcinomas to the brain with known primaries were immunostained with antibodies to CK 7, CK 20, and CAM5.2. Breast carcinoma and renal cell carcinoma also expressed as CAM5.2. CAM5.2 is a useful confirmatory stain in suspected metastatic adenocarcinoma to the brain. Unlike nonspecific AE1/3, CAM5.2 does not stain astrocytes. AE1/3 antibody should be avoided in the brain because of the common staining of both normal and neoplastic astrocytes, but CAM5.2 does not suffer this drawback and it is expressed in metastatic tumors to the brain.[16]
Kriho et al. concluded that normal and malignant astrocytes can be positive with nonspecific CKs AE1/3 along with GFAP but not with monoclonal antibody CAM5.2. This is due to the presence of keratin polypeptides in these tissues.[17]
An understanding of the pathology of CNS tumors plays a vital role in the management of patients and in clinical and biological research. Although CT and magnetic resonance imaging allow accurate localization of intracranial and spinal lesions, and often serve as a very good guide to the nature of the lesion, the final diagnosis of a tumor relies almost exclusively on histological evaluation of tissue taken at biopsy or autopsy. Pathology, radiology, and clinical evaluation all play a key role in the diagnosis and prognosis of tumors of the nervous system. An accurate diagnosis of brain tumors is usually possible after a careful assessment of routine microscopic features with sufficient clinical and radiological information.
Conclusion
High-grade gliomas such as GBM are fairly encountered in routine surgical neuropathology and it is crucial to differentiate them from metastatic tumors. Categorization is more problematic in such cases due to various parameters including the presence of necrosis and small sample size due to stereotactic biopsies. From the present study, it is concluded that IHC is a valuable technique and the best effective combination in differentiating high-grade gliomas from metastatic tumors of CNS are GFAP and CAM5.2.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest.
References
- Diagnostics of Central Nervous System Metastatic Disease. Available from: http://www.onk.ns.ac.rs/archive/vol14/PDF
- [Google Scholar]
- New immunohistochemical markers in the evaluation of central nervous system tumors: A review of 7 selected adult and pediatric brain tumors. Arch Pathol Lab Med. 2007;131:234-41.
- [Google Scholar]
- Some recent advances in neuro-oncology with particular reference to newer techniques for diagnosis and prognostication. Indian J Pathol Microbiol. 1990;33:195-209.
- [Google Scholar]
- Identification of glial fibrillary acidic protein by the immunoperoxidase method in human brain tumors. J Neuropathol Exp Neurol. 1977;36:645-52.
- [Google Scholar]
- Keratin intermediate filament expression in astrocytic neoplasms: Analysis by immunocytochemistry, western blot, and northern hybridization. Mod Pathol. 1993;6:342-7.
- [Google Scholar]
- Evaluation of epithelial and keratin markers in glioblastoma multiforme: An immunohistochemical study. Arch Pathol Lab Med. 1999;123:917-20.
- [Google Scholar]
- Immunohistochemistry of central nervous system tumors. Its contributions to neurosurgical diagnosis. J Neurosurg. 1984;60:1121-33.
- [Google Scholar]
- An immunohistochemical study of human central and peripheral nervous system tumors, using monoclonal antibodies against neurofilaments and glial filaments. Hum Pathol. 1984;15:248-57.
- [Google Scholar]
- Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984;37:975-83.
- [Google Scholar]
- Immunohistochemical localization of glial fibrillary acidic protein in human glial neoplasms. Cancer. 1980;45:484-94.
- [Google Scholar]
- An immunocytochemical comparison of glial fibrillary acidic protein, S-100p and vimentin in human glial tumors. J Neurooncol. 1990;8:33-40.
- [Google Scholar]
- Metastatic adenocarcinoma to the brain: An immunohistochemical approach. Hum Pathol. 1997;28:938-43.
- [Google Scholar]
- Keratin expression in astrocytomas: An immunofluorescent and biochemical reassessment. Virchows Arch. 1997;431:139-47.
- [Google Scholar]






