Translate this page into:
How Long does Denervation Take in Poliomyelitis? Or is it a Lifetime?”
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objective:
This study aims to determine the period of reinnervation in patients with poliomyelitis. This research was conducted to identify the appearance of denervation potentials in patients with poliomyelitis as indicators for reinnervation.
Materials and Methods:
A total of 246 male patients with poliomyelitis were assessed electrophysiologically between 1988 and 2007. The mean age was 22.8 (18–42). It has been an average of 19.9 ± 4.9 years since the beginning of complaints from the patients.
Results:
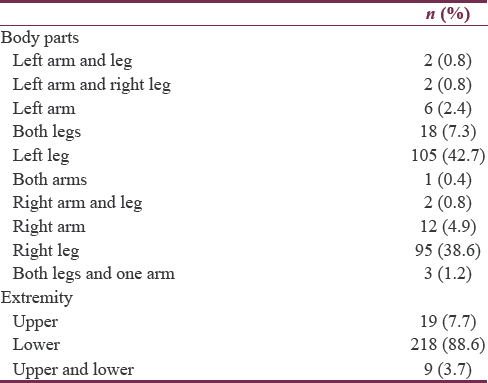
The patients had no complaints of newly developing muscle weakness, fatigue, muscle and joint pain, and difficulties in breathing and swallowing. Neurological examinations revealed the absence of myotomal pain and sensory loss. Upon assessment of the patients’ limbs, the following findings were revealed: two patients had left upper and lower limb involvement, two patients had left upper and right lower limb involvement, 6 patients had left upper limb involvement, 12 patients had both lower limb involvement, 105 patients had left lower limb involvement, 1 patient had both upper limb involvement, 2 patients had right lower and upper limb involvement, 12 patients had right upper limb involvement, 6 patients had both lower limb involvement, 95 patients had right lower limb involvement, and 3 had all the three extremities affected. The needle electromyography revealed the presence of denervation potentials in 25.2% (62) of the patients.
Conclusion:
When poliovirus attacks the motor neuron, this neuron may be completely destroyed, damaged, or unaffected. Reinnervation occurs when nearby functioning motor units send out terminal axon sprouts to reinnervate the damaged muscle fibers. As a consequence of poliomyelitis, several muscle fibers become atrophic and fibrotic, but others continue to survive. This study showed that patients with a history of poliomyelitis experienced denervation with subsequent reinnervation for many years.
Keywords
Denervation
electromyography
polio
poliomyelitis
reinnervation
INTRODUCTION
Poliomyelitis (polio) is a disease of the anterior horn motor neurons that occurs as a consequence of a generalized viral infection, causing acute lower motor neuron dysfunction. With the widespread use of an oral polio vaccine, the ratio of acute poliomyelitis markedly decreased.[1]
Global efforts to eliminate all cases of polio are ongoing. The number of poliomyelitis cases dropped from 350,000 in 1988 to 125 in 2015.[2] The transmission of poliovirus has been interrupted in all countries except for Afghanistan, Nigeria, and Pakistan. On the other hand, the outbreak of polio in the East and Central Africa as well as the Middle East between 2013 and 2014 was due to the introduction of wild polio virus.[3]
The last polio cases were reported in Turkey on November 1998. However, as Turkey has a long border with Iraq and Syria, a cross-border contamination occurred. Therefore, risk measures against polio were continued.[4]
Acute poliomyelitis destroys the anterior horn neurons, causing denervation of the muscle fibers within the motor unit. On the other hand, reinnervation serves as a compensatory mechanism against the cell loss. Thus, collateral sprouting and muscle hypertrophy occur.[5]
Previous studies reported that very old cases of polio were identified through neurophysiological examinations. Electromyography (EMG) revealed typical findings of a chronic neurogenic disease at different levels. These findings generally include a nonsymmetrical muscle distribution. Denervation potentials, such as fibrillation and positive sharp waves, were also observed.[6]
The length of time necessary for the reinnervation occurs in polio patients is still unknown. Thus, this study was conducted to identify the denervation potentials in patients with poliomyelitis as indicators for reinnervation.
MATERIALS AND METHODS
Patients
Data from this cohort study were used and reviewed retrospectively. A total of 246 male patients with a history of polio were investigated in the laboratory. Patients with a history of acute polio were included in this study, whereas patients with other comorbidities (previous infection, diabetes, radiculopathy, etc.) that might cause bias in the nerve conduction study/EMG results were excluded. The patients were referred to various clinics of the Gülhane Military Medical Academy Haydarpaşa Training Hospital and were evaluated by a neurologist; the patients’ ages varied between 18 and 42 years. All participants provided a written informed consent. Table 1 summarizes the patients’ data. The patients answered a standardized questionnaire and were neurologically examined.

Methods
The majority of EMG studies were done using the Nihon-Kohden Neuropack four Minipack (Tokyo, Japan) with the use of concentric needle electrodes. In some cases, the Medelec Multimedia EMG/EP Synergy (England) was used.
Nerve conduction velocity study
Motor and sensory conduction velocities in the median and ulnar nerves of the upper limb were studied. On the other hand, the sensory conduction velocities in the peroneal, posterior tibial, motor, and sural nerves of the lower limb were also observed. A percutaneous stimulation of the nerve was carefully performed. The skin temperature of both limbs was measured and maintained above 31°C as recommended.
Concentric needle electromyography
The concentric needle EMG (CNEMG) electrodes were used in this study. EMG signals from the muscles of the affected and unaffected limbs of polio patients were recorded. Spontaneous resting activity was assessed visually. Results showed decreased spontaneous activity and inconsistency in the interference pattern of amplitudes, durations, and phases of the motor unit action potentials (MUAPs). The results of the experimental data were compared with the normal values obtained from our EMG laboratory.
Fibrillations, positive sharp waves, and complex repetitive discharges were accounted for and considered abnormal when these potentials are observed in more than one site. The experimental data were compared with the normal values obtained from our EMG laboratory.
The proportion of patients due to the use of assistive devices for movement ability and muscle weakness than had applied EMG.
Creatine kinase
The determination of creatine kinase (CK) was made at the GATA Haydarpaşa Training Hospital. In this procedure, patients’ blood samples were obtained in the morning prior to needle EMG. Patients were prohibited from doing strenuous exercise or exhausting activities on the same day prior to the test. Patients with a potentially spurious cause of elevated CK (such as recent EMG, medication, intramuscular injection, and muscular trauma) were excluded. The CK used for data analysis was the initial determinant during or after patient's clinical diagnosis was established.
Statistical analysis
A comparison of the statistical differences between the two groups was done using the Mann–Whitney (Wilcoxon), Chi-square, and regression tests. The maximum level of significance was P = 0.05. The mean values, standard error of the mean, and confidence intervals were calculated using conventional formulas. Standard software (SPSS, Chicago, IL, USA) was used in the statistical analysis.
RESULTS
The following are the results of the study: 19 (7.7%) patients had upper limb involvement, 218 (88.6%) patients had lower limb involvement, and 9 (3.7%) patients had both upper and lower limb involvement. Table 2 summarizes the patients’ data.
EMG findings
The EMG results showed classic neurogenic signs, reinnervation potential, and spontaneous activity including positive sharp waves, fibrillation, and fasciculations in the involved muscle. In patients with poliomyelitis, an increase in MUAP amplitude, duration, and polyphasia was observed during CNEMG.
Spontaneous activities were noted in the EMG, and MUAPs are summarized in Table 3. The MUAPs in the majority of the examined patients indicate the presence of anomalies. Continuing denervation potentials were identified in 25.2% of the patients. An increase in the duration of voluntary MUAPs and amplitude was observed during needle EMG. Additionally, polyphasia was seen in 49.2% of the patients whose muscles have been examined.

Concentrations of creatine kinase
The serum CK levels in poliomyelitis patients were between 45 and 253 (mean +1 standard deviation = 147 ± 28) IU/l. The total serum CK level was elevated (>170 IU/l) in 12 (4.9%) of the 246 patients. Patients with elevated CK concentration were all working males, with eight of them doing increased physical activities and working (physical activity scores >240). The clinical examination showed no findings related to postpolio syndrome.
DISCUSSION
The aim of this study was to identify whether long-term denervation occurs in poliomyelitis patients or not, and if ongoing, to determine the maximum denervation period. This study showed that denervation along with reinnervation continues for many years in patients previously diagnosed with polio.
In this study, the EMG findings showed an increase in the period and amplitude of MUAPs, reflecting the excessive rate of reinnervation due to the germination of regenerated neurons in the muscles previously affected by poliomyelitis. Similar evidences were determined in the study by Stålberg and Grimby.[7]
Acute polio flax paralysis exhibits the clinical characteristics of an alpha motor neuron injury. Some of the denervated muscle fibers are reinnervated by the collateral sprouting of the remaining motor axons, and thus the paralysis is healed in varying degrees. A similar type of regeneration is seen in other neurogenic conditions such as polyneuropathy and local nerve lesions. Therefore, muscle function loss is not specific to poliomyelitis but can occur in other neuropathies.[8]
The exact etiology of prolonged denervation remains unknown. However, countless theories involving immunopathogenic mechanisms, neural aging, and viral reactivation have been suggested.[9] The most reasonable hypothesis would be the excessive use of muscles for many years, excessive use of huge motor units, and excessive metabolism demand, leading to the decrease in axonal sprouting.[101112]
Polio virus damages the front horn motor neurons of the spinal cord and/or brain stem, causing weakness in the lower motor neurons and eventually paralysis. The remaining motor neurons are subjected to axonal sprouting and hypertrophy, but this is not a stable situation; they are more vulnerable to continuous reformation and premature failure, and they cannot keep the muscle strength for a long time. This leads to failure and degeneration of the axon germination by aging motor units, fatigue in neurons working excessively due to the increasing metabolic demand from the present neurons, and/or new muscle weakness in the current motor neurons.[13]
Progressive loss of front horn cells occurs with aging.[14] Therefore, in patients with a history of poliomyelitis, denervation-reinnervation progressing slowly with age becomes effective as it accelerates as years go by. Front horn cells decrease, rate of sprouting increase in muscle fibers becomes multiple times higher than normal. Hence, the denervation and reinnervation occur in the major motor units.[15]
EMG shows the loss of motor units and reinnervation as typical symptoms in patients who have had poliomyelitis. Initially, monitoring of reinnervation in neurons during acute attacks is done. In addition, a probable continuing process occurs due to the changes happening as the person ages.[14] In case of reinnervation by the way of collateral sprouting, the remaining motor units innervate all the denervated muscle fibers. Therefore, only a few parts of a certain motor unit can be involved in the reinnervation process within its own territory.[16]
Patients who have had poliomyelitis generally have different levels of chronic neurogenic evidences in different muscles typically with an asymmetric distribution. Decrease in chronic reinnervation and motor unit recruitment is seen. In addition to these changes, continuing denervation is evidenced by the presence of fibrillation and fasciculation potentials.[17]
It has been reported that denervation potentials is also seen in the unaffected muscle fibers of poliomyelitis patients. Furthermore, in the absence of new clinical evidence, motor unit loss due to aging can occur in poliomyelitis patients. This can indicate the failure of compensatory mechanisms in people with poliomyelitis history.[18]
Leg muscles of poliomyelitis patients are much more affected than their arm muscles. If the chest and truncal muscles are affected, the disease may progress and cause tetraplegia. Similar to the previous studies, most of our patients had lower-extremity involvements.[19]
The long-term studies showed that the progression of poliomyelitis decreases as the patient's health status improves; fasciculation develops but symptoms of the upper motor neuron symptoms do not improve. The relationship of rising CK levels has not been fully revealed.[20]
A variety of medical conditions may occur in poliomyelitis patients in the upcoming years, and the risk of orthopedic and other conditions increases.[21] The electrophysiological evidences indicating denervation continuing in 25% of the patients are shown in this study. Better knowledge on these matters will probably contribute to better treatment of these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Atypical motor neuron disorders. In: Preston DC, Shapiro BE, eds. Electromyography and Neuromuscular Disorders. London: Elsevier Saunders Inc; 2013. p. :432-48.
- [Google Scholar]
- 2015. Weekly Epidemiological Record. 24 April, 2015, 90th Year. No. 17. 90:169-84. Available from: http://www.who.int/entity/wer/2015/wer9017.pdf?ua=1
- UNICEF (2015). Data companion to the annual report of the Executive Director. Available from: http://www.unicef.org/about/execboard/files/EDAR_Data_Companion_2015-15Jun2015-EN.pdf (in http://www.un.org/en/ecosoc/qcpr/pdf/qcpr2016-list_of_sources.pdf)
- Comparison between concentric needle EMG and macro EMG in patients with a history of polio. Clin Neurophysiol. 1999;110:1900-8.
- [Google Scholar]
- Electromyographic and morphological functional compensation in late poliomyelitis. Muscle Nerve. 1990;13:165-71.
- [Google Scholar]
- Dynamic electromyography and muscle biopsy changes in a 4-year follow-up: Study of patients with a history of polio. Muscle Nerve. 1995;18:699-707.
- [Google Scholar]
- How to interpret normal electromyographic findings in patients with an alleged history of polio. J Rehabil Med. 2004;36:169-76.
- [Google Scholar]
- Late denervation in patients with antecedent paralytic poliomyelitis. N Engl J Med. 1987;317:7-12.
- [Google Scholar]
- A long-term follow-up study of patients with post-poliomyelitis neuromuscular symptoms. N Engl J Med. 1986;314:959-63.
- [Google Scholar]
- A syndrome post-polio. In: Umphred DA, ed. Neurological rehabilitation (4th ed). Sao Paulo: Manole Ltd; 2004. p. :608-626.
- [Google Scholar]
- Late effects of polio: Critical review of the literature on neuromuscular function. Arch Phys Med Rehabil. 1991;72:923-31.
- [Google Scholar]
- An 8-year longitudinal study of muscle strength, muscle fiber size, and dynamic electromyogram in individuals with late polio. Muscle Nerve. 1998;21:1428-37.
- [Google Scholar]
- Dynamic changes in muscle structure and electrophysiology in late polio with aspects on muscular trainability. Scand J Rehabil Med Suppl. 1994;30:33-44.
- [Google Scholar]
- Scanning EMG in normal muscle and in neuromuscular disorders. Electroencephalogr Clin Neurophysiol. 1991;81:403-16.
- [Google Scholar]
- Radiculopathy and motor neuron disorders. In: Blum AS, Rutkove SB, eds. The Clinical Neurophysiology Primer. Totowa, New Jersey: Humana Press Inc; 2007. p. :289-98.
- [Google Scholar]
- Electrophysiological findings in a cohort of old polio survivors. J Peripher Nerv Syst. 2006;11:241-6.
- [Google Scholar]
- Late effects of poliomyelitis – An update on fatigue and weakness. West J Med. 1992;157:663.
- [Google Scholar]
- Radiculopathy and motor neuron disorders. In: Blum AS, Rutkove BS, eds. The Clinical Neurophysiology Primer. Totowa, NJ: Humana Press Inc; 2007. p. :289-98.
- [Google Scholar]
- The survey of aging late effects of Korean polio survivors: Preliminary study by telephone interview. PM R. 2013;5(Supp 9):179.
- [Google Scholar]






