Translate this page into:
Crystalloids versus hydroxyethyl starch (130/0.4) in patients undergoing decompressive craniectomy for isolated traumatic brain injury: A prospective randomized controlled trial
*Corresponding author: Kirandeep Kaur, Department of Anesthesiology, Maharishi Markandeshwar Institute of Medical Sciences and Research, Ambala, Haryana, India. kirandeep9150@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Hemachandiran R, Jangra K, Barik AK, Kaur K, Kumar A, Panda NB, et al. Crystalloids versus hydroxyethyl starch (130/0.4) in patients undergoing decompressive craniectomy for isolated traumatic brain injury: A prospective randomized controlled trial. J Neurosci Rural Pract. 2025;16:68-75. doi: 10.25259/JNRP_326_2024
Abstract
Objectives:
The use of hydroxyethyl starch (HES) during neurosurgical patients is debatable. Previous literature suggests that HES interferes with coagulation parameters and kidney function tests. However, most of the evidence was extrapolated from studies including critically sick intensive care unit patients. Thus, we planned to compare crystalloids and HES for coagulation parameters in isolated traumatic brain injury (TBI) patients undergoing decompressive craniectomy.
Materials and Methods:
The prospective randomized controlled trial included the American Society of Anesthesiologists I and II adult patients (18– 65 years) with isolated TBI posted for decompressive craniectomy. Patients were randomized equally into two groups (Group Cs and Group Co). Group Cs received crystalloids (PlasmaLyte-A, Beiffe Medital S.A, Huesca, Spain) and Group Co received a combination of crystalloids (PlasmaLyte-A) and colloids (HES 6% 130/0.4, Voluven®, Fresenius Kabi Ind Pvt. Limited). Coagulation parameters using sonoclot, intraoperative hemodynamics, brain relaxation score (BRS), blood loss, serum lactate, electrolytes, total intravenous fluids used, post-operative kidney function, and modified Rankin score (mRS) were compared in two groups. The continuous data were presented as mean ± Standard deviation, and categorical data as frequency (percentage). Inferential statistics such as the Chi-square test/Fischer Exact test and independent t-test were applied to compare the two groups.
Results:
A total of 60 patients were analyzed with 30 patients in each group. Sonoclot parameters were comparable in the two groups at baseline and the end of surgery. There were no differences in BRS, electrolytes, serum lactate, blood loss, urine output, use of vasopressors, post-operative parameters including urea, creatinine, and median mRS between the groups.
Conclusion:
We conclude that HES (130/0.4) may be safely given intraoperatively in limited dosages in isolated head injury patients as it does not affect coagulation parameters. Other intraoperative variables including hemodynamics, BRS, total blood loss, the total volume of fluids used, serum electrolytes, and serum lactate, urine output, and mRS were comparable between the groups. Short-term use of modern colloids was not associated with post-operative renal dysfunction.
Keywords
Coagulation
Colloids
Crystalloid
Neurosurgery
Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is associated with significant morbidity and mortality.[1] Secondary brain injuries including hypotension and compromised cerebral perfusion are associated with an adverse neurological outcome.[2] This is attributed to the severity of initial trauma, the use of osmotherapy, and intraoperative blood loss. Intraoperative fluid management in neurosurgical patients is critical to maintain systemic and cerebral hemodynamics and providing favorable surgical conditions. Emerging evidence suggests the importance of intraoperative fluid to impact post-operative outcomes.[3,4] However, the ideal fluid in neurosurgical patients is still debatable.[1]
To achieve similar hemodynamic goals, the amount of crystalloid required is much more than the colloid.[5,6] In high dosage, crystalloids may result in fluid overload, pulmonary and interstitial edema, dilutional coagulopathy, platelet dysfunctions, cerebral edema, rise in intracranial pressure, and also aggravate the neuroinflammatory response, leading to poor tissue oxygenation, and even organ failure.[7-9] In contrast, the colloid solutions cause a rapid intravascular volume expansion, maintenance of oncotic pressure, and less tissue and pulmonary edema.[10] Hydroxyethyl starch (HES) is one of the commonly used colloids. There is a potential risk for acute kidney injury (AKI) and derangement in the coagulation profile with the use of HES.[10-12] However, the evidence regarding AKI was mainly extrapolated from the intensive care set-up where HES was administered in critically ill patients.[13] The coagulation derangements were mainly observed with the use of high and medium molecular weight HES.[14] Current evidence suggests that modern HES is not associated with an increased risk of AKI or coagulation abnormalities in a variety of critically ill patients.[15,16] However, there is a need to generate an evidence pertaining to the intraoperative use of HES.[17]
Hence, we planned this prospective randomized trial with the primary objective of comparing crystalloids (PlasmaLyte-A) and mixed fluids (PlasmaLyte-A and HES) on coagulation parameters. Other intraoperative variables including hemodynamics, brain relaxation scores (BRS), total blood loss, the total volume of fluids and blood used, serum electrolytes, and serum lactate were also compared. The renal function tests were done, and the AKI Network (AKIN) score was calculated at 24 h and 48 h in the postoperative period. Patient outcome at discharge was compared in two groups using a modified Rankin score (mRS).
MATERIALS AND METHODS
The prospective double-blind randomized trial conforms to the norms of the Declaration of Helsinki. The patients were enrolled after obtaining approval from the Institutional Ethics Committee (NK/6977/MD/151) and obtaining written informed consent from the next of kin. The study included the American Society of Anesthesiologists (ASA) I and II adult patients (18–65 years) with moderate-to-severe isolated TBI posted for decompressive craniectomy. The patients with associated extracranial injuries, ASA III and above, pre-operative coagulopathy, history of consumption of non-steroidal anti-inflammatory drugs, antiplatelets, and anticoagulants within the past two weeks, significant intraoperative blood loss (more than one blood volume), and pregnant patients were excluded from the study.
Patients were randomized equally into two groups (Group Cs and Group Co) using Random Allocation software and allocation concealment was done using the Serially numbered opaque sealed envelope technique. The surgeons who assessed the BRS and the anesthesiologist who analyzed the outcome parameters were blinded to the study fluid.
Group Cs received crystalloids (PlasmaLyte-A, Beiffe Medital S.A, Huesca, Spain) and Group Co received a combination of crystalloids (PlasmaLyte-A) and colloids (HES 6% 130/0.4, Voluven®, Fresenius Kabi Ind Pvt. Limited). Baseline investigations, including hemogram, coagulation, kidney function tests, serum electrolytes, arterial blood gases (ABG), and Glasgow coma scale (GCS) score, were done in both groups.
Standard intraoperative ASA monitors including oxygen saturation (SpO2), electrocardiography, and non-invasive blood pressure were attached. Two wide-bore intravenous cannulas and an arterial line for beat-to-beat monitoring were secured. Intravenous anesthesia technique as per institutional protocol was followed in all the patients using injections of fentanyl, propofol, and vecuronium. Patients were maintained with oxygen and air (1:1 ratio). In the Cs group PlasmaLyte-A and Co group, HES was started as the initial fluid @ 5–10 mL/kg depending on the hydration status, as guided by the pleth variability index, using Masimo SET® Pulse Oximetry (Masimo Corporation, Irvine, CA) with trigger to treat hypotension as per standard Brain trauma foundation guidelines.[18] For maintenance, in Group Cs, PlasmaLyte-A was continued, while in Group Co, HES was given till the total volume of HES reached 20 mL/kg or 1 L (whichever was lower). PlasmaLyte-A was administered for further maintenance [Figure 1]. Blood and blood products were transfused if the blood loss approached maximum allowable limits.[19] The sample size was calculated based on the findings from the previous study. According to an article by Golparvar et al., the mean change in reaction time in the crystalloid group was 20.61 ± 26.46s.[20] A sample size of 26 in each group was calculated using OpenEpi Toolkit with 80% power of the study, 95% confidence interval, and 5% level of significance for an effect size of 0.8. Anticipating 10% dropouts, we recruited 30 patients in each group with a total of 60 patients.
- Consort diagram. Cs: Crystalloid, Co: Colloid.
Intraoperative parameters, including continuous monitoring for systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure, heart rate (HR), SpO2, and end-tidal carbon dioxide (EtCO2), were noted. BRS (before dural opening), total blood loss, total volume of I.V. fluid, use of vasopressors, and urine output were recorded. To avoid inter-rater bias for brain relaxation, a 4-point objective scale was used. Grade I was the completely relaxed brain, lying below the inner table of the skull. Grade II included a satisfactorily relaxed brain touching the inner table and pulsating well. In grade III, the brain was firm/tense but lying between the inner and outer table with weak pulsations. In grade IV, the brain was tight and bulging out of the outer table of the skull with faint or no pulsations. ABG was done at various time points including, baseline (before the commencement of surgery), at the start of blood loss, and at the end of surgery. Serum lactate and clotting function (Sonoclot) were assessed at baseline and the end of surgery. The Sonoclot parameters included activated clotting time (ACT), clot rate (CR), and peak amplitude (PA). Post-operative parameters including blood urea, and serum creatinine were repeated at 24 and 48 h for calculating the AKIN score, and mRS was assessed at the time of discharge.
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows, Version 22.0. (Armonk, NY: IBM Corp). The continuous data were presented as mean ± standard deviation, and categorical data as frequency (percentage). The normality of the data was assessed using the Shapiro-Wilk test. The Chi-square test/Fischer Exact test was applied to find out the significant association between groups and categorical variables such as gender and BRS. An independent t-test was applied to compare hemodynamic parameters, blood loss, mean colloid volume, blood transfusion, urine output, blood urea, and serum creatinine. For GCS and mRS comparisons between the two groups, the Mann–Whitney U-test was applied, and data were presented in median and IQR. A two-sided P < 0.05 was considered statistically significant with a 95% confidence interval.
RESULTS
A total of 60 patients were analyzed with 30 patients in each group, Cs and Co [Figure 2]. The two groups were comparable in demographics and baseline characteristics [Table 1].
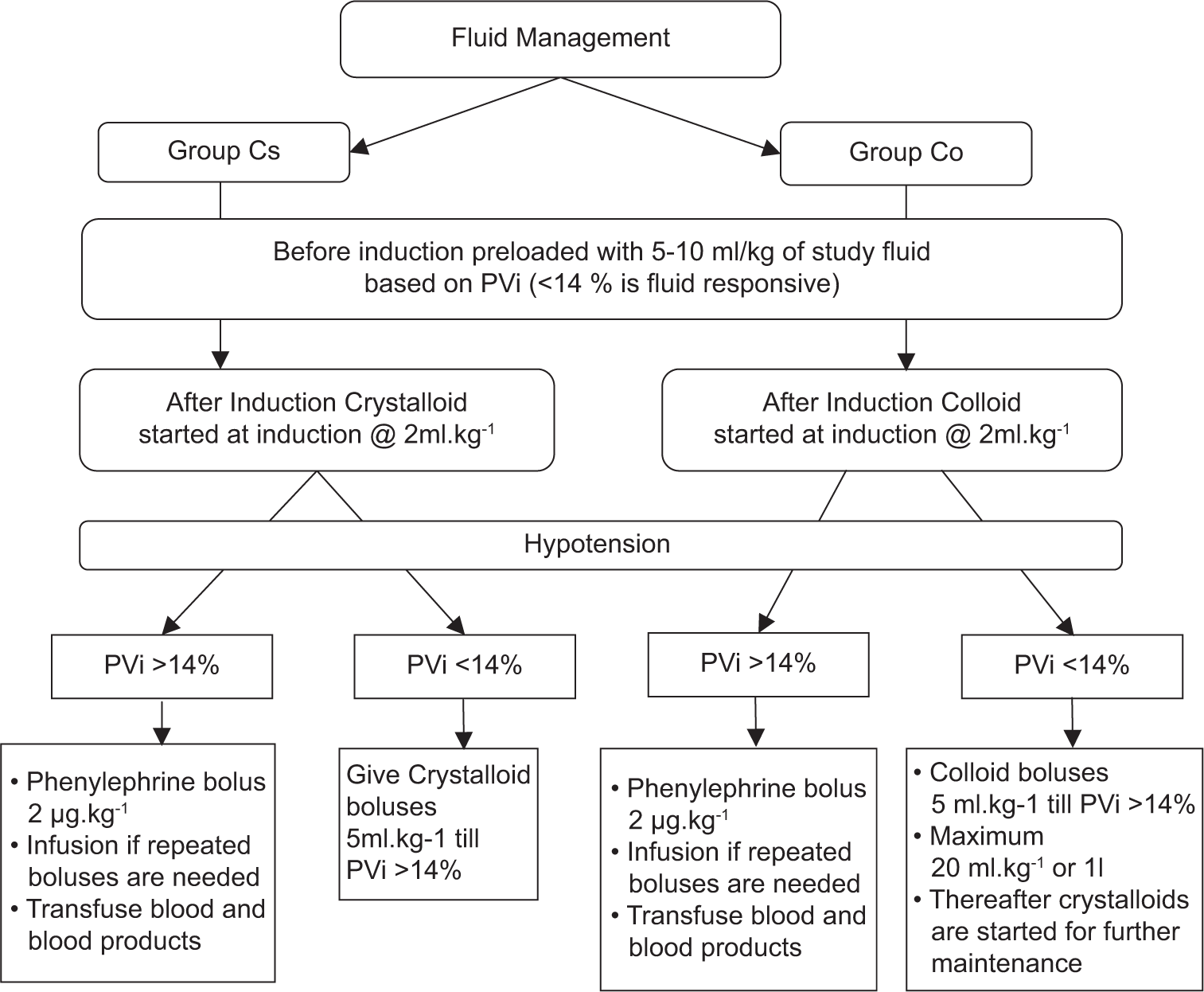
- Protocol for fluid management in groups crystalloid (Cs) and colloid (Co). PVi: Pleth variability index.
| Parameters | Group Cs (n=30) | Group Co (n=30) | P-value |
|---|---|---|---|
| Age, years | 44.30±15.36 | 39.10±12.98 | 0.162 |
| Weight, kg | 68.27±9.97 | 70.23±7.72 | 0.396 |
| Male, n (%) | 24 (80) | 28 (93.3) | 0.254 |
| Female, n (%) | 06 (20) | 02 (6.7) | |
| ASA, n (%) | |||
| I | 24 (80) | 28 (93.3) | 0.254 |
| II | 06 (20) | 02 (6.7) | |
| GCS, (Median [IQR]) | 9 (5–14) | 9 (6–11) | 0.840 |
| Platelet (mm3) | 181133.33±68741.76 | 165353.33±52410.65 | 0.322 |
| Serum sodium (meq/L) | 137.05±2.97 | 138.56±3.91 | 0.096 |
| Serum potassium (meq/L) | 4.03±0.49 | 4.06±0.42 | 0.792 |
| Serum chloride (meq/L) | 102.33±4.18 | 102.83±4.50 | 0.657 |
| Urea (mg/dL) | 26.60±8.02 | 30.25±8.37 | 0.090 |
| Creatinine (mg/dL) | 0.75±0.28 | 0.81±0.19 | 0.387 |
| PT (s) | 14.22±1.36 | 14.28±1.24 | 0.844 |
| INR% | 1.05±0.09 | 1.06±0.07 | 0.561 |
| PTI (s) | 91.80±6.74 | 90.14±6.13 | 0.323 |
| aPTT (s) | 29.36±3.20 | 28.69±3.67 | 0.459 |
Data are presented as mean±SD and number (percentage). GCS: Glasgow coma scale, PT: Prothrombin time, INR: International normalized ratio, PTI: Prothrombin index, aPTT: Activated partial thromboplastin time, ASA: American society of anesthesiologists, SD: Standard deviation, IQR: Interquartile range, Cs: Crystalloid, Co: Colloid.
The intraoperative HR remained comparable between the two groups throughout the surgery except at 50 min after the start of surgery where, in Group Cs, it was 75.73 ± 17.4 and, in Group Co, it was 83.7 ± 9.34 with a statistically significant difference between them (P < 0.030) but the finding was clinically insignificant [Figure 3a]. SBP and DBP gradually decreased in the two groups, but the difference was not statistically significant [Figure 3b1 and b2]. Intraoperative hypotension was observed in three patients in Group Cs and two patients in Group Co, which was statistically comparable. The average intraoperative blood loss in Group Cs was 319.50 ± 162.53 and 348.00 ± 126 mL in Group Co and their difference was not statistically significant.
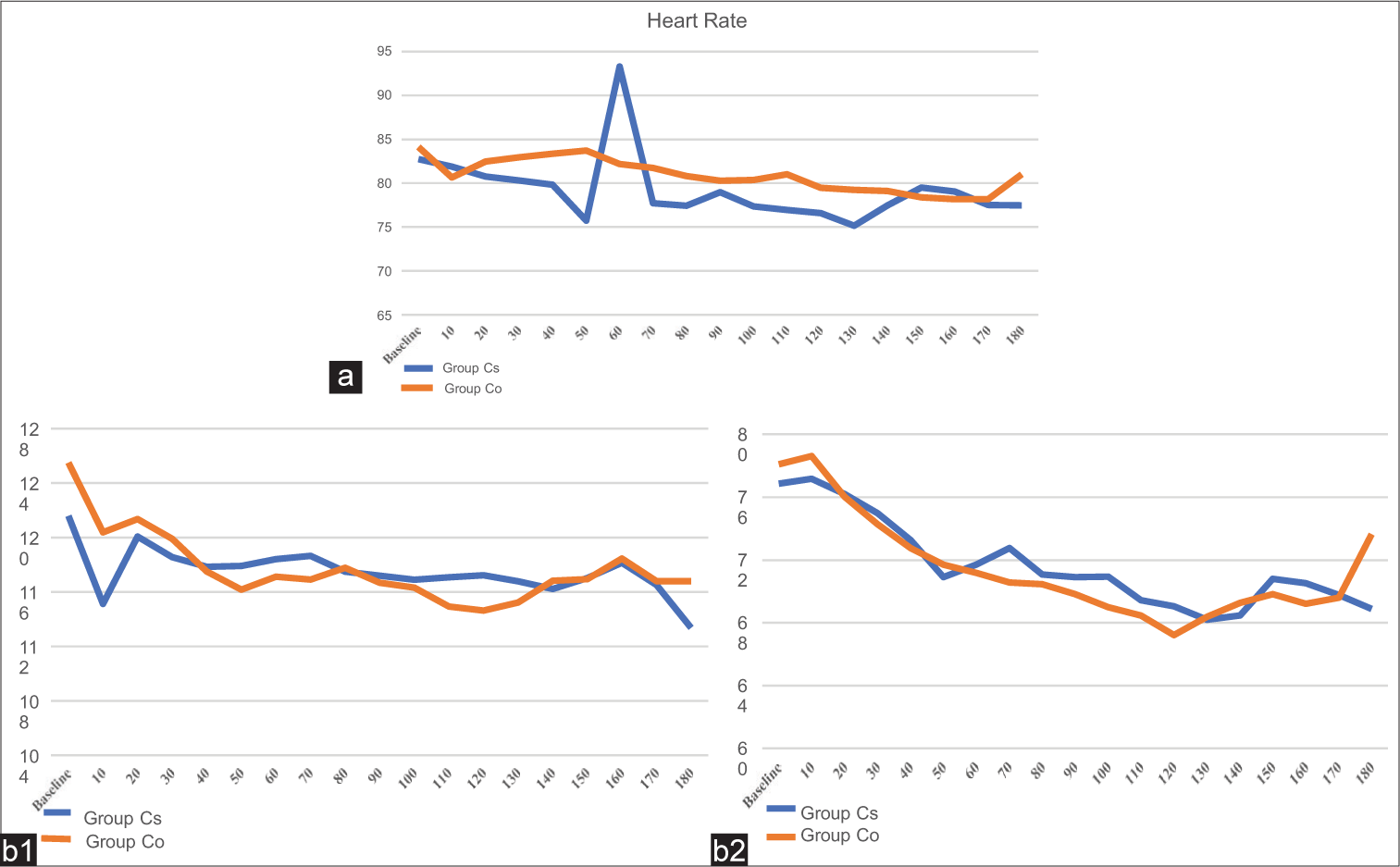
- (a) Mean intraoperative heart rate in two groups. (b1) Mean intraoperative systolic blood pressure in two groups. (b2) Mean intraoperative diastolic blood pressure in two groups. Cs: Crystalloid, Co: Colloid.
Sonoclot parameters, including ACT, CR, and PA, were comparable in the two groups at baseline and at the end of surgery [Table 2]. There were no differences in BRS, intraoperative serum electrolytes, serum lactate, blood loss, urine output, total fluid infused, post-operative parameters, including urea and creatinine, and median mRS between the groups [Table 2].
| Parameters | Group Cs (n=30) | Group Co (n=30) | P-value |
|---|---|---|---|
| Activated clotting time (s) | |||
| Baseline | 134.07±22.56 | 132.53±36.97 | 0.847 |
| End of surgery | 136.13±23.44 | 138.90±40.69 | 0.748 |
| Clot rate (units/min) | |||
| Baseline | 30.34±9.76 | 30.39±12.64 | 0.986 |
| End of surgery | 30.34±10.81 | 32.11±12.81 | 0.567 |
| Platelet activity (units) | |||
| Baseline | 3.15±1.27 | 2.51±1.31 | 0.060 |
| End of surgery | 2.54±1.46 | 2.58±1.26 | 0.903 |
| Brain relaxation score | |||
| Completely relaxed, n (%) | 20 (66.7) | 12 (40) | 0.092 |
| Satisfactorily, n (%) | 09 (30) | 16 (53.3) | |
| Firm, n (%) | 01 (3.3) | 02 (6.7) | |
| Serum sodium (mEq/L) | |||
| Baseline | 137.38±6.28 | 137.88±6.2 | 0.756 |
| Start of blood loss | 134.48±5.75 | 136.74±3.22 | 0.421 |
| End of surgery | 136.39±5.17 | 137.92±5.85 | 0.290 |
| Serum potassium (mEq/L) | |||
| Baseline | 3.65±0.68 | 3.56±0.73 | 0.629 |
| Start of blood loss | 3.15±0.5 | 3.3±0.43 | 0.573 |
| End of surgery | 3.75±0.82 | 3.63±0.62 | 0.516 |
| Serum calcium (mEq/L) | |||
| Baseline | 0.92±0.13 | 0.93±0.12 | 0.757 |
| Start of blood loss | 0.89±0.12 | 0.92±0.09 | 0.559 |
| End of surgery | 0.98±0.49 | 0.91±0.09 | 0.440 |
| Serum chloride (mEq/L) | |||
| Baseline | 103.87±20.42 | 107.87±6.76 | 0.313 |
| Start of blood loss | 106.86±3.72 | 110.4±3.51 | 0.081 |
| End of surgery | 107.83±5.88 | 108.7±6.06 | 0.576 |
| Serum lactate (mmol/L) | |||
| Baseline | 1.36±1.04 | 1.61±1.32 | 0.421 |
| Start of blood loss | 1.26±1.11 | 1.63±0.71 | 0.504 |
| End of surgery | 1.49±0.90 | 1.56±1.09 | 0.805 |
| Total blood loss (mL) | 319.50±162.53 | 348.00±126.61 | 0.452 |
| Urine output (mL) | 409.33±66.48 | 432.33±110.25 | 0.812 |
| Total fluid given (mL) | 1033.33±347.73 | 1180.00±397.10 | 0.133 |
| Post-operative blood urea (mg/dL) | |||
| 24 h | 30.00±7.96 | 33.62±8.14 | 0.087 |
| 48 h | 28.83±6.48 | 30.2±7.32 | 0.447 |
| Post-operative serum creatinine (mg/dL) | |||
| 24 h | 0.81±0.16 | 0.88±0.16 | 0.089 |
| 48 h | 0.76±0.22 | 0.81±0.16 | 0.332 |
| mRS, Median (IQR) | 2 (1-3) | 1 (1-2) | 0.064 |
Data are presented as mean±SD and number (percentage). mRS: modified Rankin score, IQR: Interquartile range, SD: Standard deviation, Cs: Crystalloid, Co: Colloid.
DISCUSSION
In the present study, we did not observe a statistically significant difference in the coagulation parameters using sonoclot at the end of surgery compared to the baseline values. Our results were different from the findings of Golparvar et al. who reported a reduction in the speed of clot formation and an increase in clot lysis in the hetastarch group compared to the saline group used in brain tumor surgeries.[20] However, they have used hetastarch (200), which has a higher molecular weight and molar substitution than tetrastarch (HES, 130/0.4) used in the current study. There are a few other studies, in vitro and healthy volunteers, that have also shown the adverse effects of HES on coagulation.[21,22] These studies were conducted either in vitro or using higher doses of various HES formulations which could have been a reason for the contrasting results. However, the literature on neurosurgical patients showed that HES is not associated with impairment in coagulation, intracranial hemorrhage, and excessive blood loss.[23-25] All these studies were retrospective in nature where the drug, dosages, and type of HES were not standardized. Hence, we have diverse literature showing variable results, and most of the studies using modern HES in lower dosages do not show adverse effects on the coagulation parameters when used intraoperatively.[14]
In the present study, intraoperative blood loss and use of blood and blood products showed no significant difference. Our results were comparable to the conclusions of the previous study, using HES intraoperatively, showing no clinically relevant difference in bleeding between colloids and crystalloids.[13] The modern colloid solutions do not affect coagulation, and indirectly, they do not contribute to more blood loss. Comparing the hemodynamic parameters, no inter or intragroup differences were found in the present study. These findings were similar to those of Golparvar et al., wherein the HR, blood pressure, EtCO2, SpO2, and temperature were similar between the groups during surgery.[20] Goodwin et al. reported an increase in urine output and improved cardiac index in patients who received colloids in burn patients.[26] This difference in the results from our study may be attributed to the differences in the subset of the study population and the fluid regimen.
Bradley et al. conducted a randomized controlled, double-blind crossover study to compare the effects of colloids (4% succinylated gelatin) and crystalloids (Sterofundin) on blood volume, renal function, and cardiac output in healthy male volunteers.[27] They concluded that small-volume colloid (500 mL) was as effective as large-volume crystalloid at expanding blood volume and increasing cardiac output (1.5 L). The results were in line with the present study. We also used colloids in a limited amount, which did not affect the hemodynamics significantly.
In the present study, BRS was comparable in two groups. In a prospective randomized controlled trial (RCT), Xia et al. compared lactated Ringer’s solution (RL) and 6% HES (130/0.4) to determine brain relaxation and cerebral metabolism in patients undergoing supratentorial tumor resection. It was found that there was no significant difference in BRS in both HES and RL groups.[28] The results were concordant with the present study. BRS was similar in both the groups as the fluid movement inside the brain is predominantly governed by the osmolarity and we have not used hypo-osmolar fluid in either group.
Serum lactate levels and serum electrolytes remained within the normal range in either group.
Similarly, Heßler et al., concluded that goal-directed colloid administration in the intraoperative period reduced the risk of fluid overload and accumulation, thus hampering the microcirculation.[13] In the present study, we have used goal-directed fluid therapy where the intravascular volume was optimally managed maintaining adequate microcirculation.
The renal parameters were within the normal range in both groups with no significant difference between them. The results were similar to a study done in cardiothoracic surgery, suggesting low-volume (<1 L) use of colloid was not associated with acute kidney dysfunction.[29] In the recent past, two RCTs were conducted in the intensive care unit setup, namely, the crystalloid versus hydroxyethyl starch trial (CHEST) [6% HES 130/0.4 vs. 0.9% normal saline (NS)] and 6S (6% HES 130/0.42 in Ringer’s acetate versus Ringer’s acetate) trials which found that tetrastarches increased the use of dialysis and blood transfusion.[13,30] However, these trials were conducted on critically ill patients with severe sepsis, and extrapolation of results to intraoperative use in acute trauma cannot be justified. In a review article by Heßler et al., it was summarized that there was no difference in the rate of AKI between colloid (6% HES 130/0.4) and crystalloid.[13] In another review article, Westphal et al. concluded that newer-generation HES has no adverse effect on the renal profile.[31] The safety of modern HES has been stated by Jungheinrich et al. in patients even with mild to severe renal impairment provided urine output is preserved. The authors used a single dose of 500 mL of 6% HES 130/0.4 in their study.[32]
The neurological outcome at discharge (assessed by mRS) was comparable in both groups. An animal study conducted by Feng et al. observed that HES (130/0.4) markedly attenuated inflammatory markers including tumor necrosing factor-alpha, interleukin-6, intercellular adhesion molecule-1 mRNA, nuclear factor-kappa B, and downregulated toll-like receptors 2 and 4.[33] These findings suggest that HES has the potential to inhibit neuro-inflammation secondary to brain injury but failed to show improvement in the clinical outcome. Even the current clinical study also failed to show any difference in the outcome of the two groups.
In the present study, we observed that intraoperative use of low-dose modern HESs (6% HES, 130/0.40) did not affect the coagulation parameters in patients with isolated TBI as compared to crystalloid. The hemodynamic parameters and BRS were comparable between crystalloids and HES groups. None of the patients developed AKI as measured by AKIN criteria among the two groups and outcome at discharge as measured by mRS was also comparable in both groups.
The study has a few limitations. The type of fluid used in the triage area was 0.9% normal saline, which was not the study fluid. Furthermore, the amount of fluid used in pre-operative resuscitation was variable. Although this factor did not bring any significant difference in our study, still this factor can be controlled. We included patients belonging to ASA status I and II with isolated TBI. The results may vary in patients with significant systemic trauma. The use of HES in ASA III/IV grades may have a varied response depending on end-organ reserve, such as patients with organ failure may develop fluid overload and AKI. The safety of HES in pediatric patients is not yet established due to the immature renal system. In patients with moderate to severe TBI, the use of HES can accentuate trauma-induced coagulopathy. Hence, the results of the present study cannot be extrapolated in these populations. Furthermore, we did not evaluate the effects of modern HES on long-term outcomes such as functional outcomes or survival of the patients. We did not perform a subgroup analysis. A small sample size makes the findings of the study less robust. Hence, a large, multicentric trial is required to generate strong evidence for the safety of intraoperative use of newer HESs.
CONCLUSION
We conclude that the use of moderate dosages of modern starch during the intraoperative period in ASA I and II isolated TBI patients did not affect the coagulation parameters. Other intraoperative variables including hemodynamics, BRS, total blood loss, total volume of fluids and blood used, serum electrolytes, and serum lactate were comparable between the groups. Short-term use of modern colloids was not associated with post-operative renal dysfunction. Hence, modern HESs may be safely given intraoperatively in limited dosages in isolated head injury patients.
Ethical approval
The research/study approved by the Institutional Review Board at Institutional Ethics committee, Postgraduate Institute of Medical Education and Research, Chandigarh, number NK/6977/MD/151, dated November 29, 2021. Clinical Trial Registry-India (REF/2021/04/042560 H).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Perioperative management of adult traumatic brain injury. Anesthesiol Clin. 2012;30:333-46.
- [CrossRef] [PubMed] [Google Scholar]
- The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216-22.
- [CrossRef] [PubMed] [Google Scholar]
- Perioperative restrictive versus goal-directed fluid therapy for adults undergoing major non-cardiac surgery. Cochrane Database Syst Rev. 2019;12:CD012767.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641-8.
- [CrossRef] [PubMed] [Google Scholar]
- Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108:384-94.
- [CrossRef] [PubMed] [Google Scholar]
- Morbidity and mortality of crystalloids compared to colloids in critically ill surgical patients: A subgroup analysis of a randomized trial. Anesthesiology. 2018;129:1149-58.
- [CrossRef] [PubMed] [Google Scholar]
- The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115-21.
- [CrossRef] [PubMed] [Google Scholar]
- Fluid administration during abdominal surgery influences on coagulation in the postoperative period. Curr Surg. 2004;61:459-62.
- [CrossRef] [PubMed] [Google Scholar]
- Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000;28:74-8.
- [CrossRef] [PubMed] [Google Scholar]
- Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;2:CD000567.
- [CrossRef] [Google Scholar]
- Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901-11.
- [CrossRef] [PubMed] [Google Scholar]
- Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: A systematic review and meta-analysis. JAMA. 2013;309:678-88.
- [CrossRef] [PubMed] [Google Scholar]
- To use or not to use hydroxyethyl starch in intraoperative care: Are we ready to answer the 'Gretchen question'? Curr Opin Anaesthesiol. 2015;28:370-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology. 2005;103:654-60.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxyethyl starch for volume expansion after subarachnoid haemorrhage and renal function: Results of a retrospective analysis. PLoS One. 2018;13:e0192832.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of fluid treatment with hydroxyethyl starch on renal function in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2016;28:187-94.
- [CrossRef] [PubMed] [Google Scholar]
- Why hydroxyethyl starch solutions should NOT be banned from the operating room. Anaesthesiol Intensive Ther. 2014;46:336-41.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the management of severe traumatic brain injury, Fourth edition. Neurosurgery. 2017;80:6-15.
- [CrossRef] [PubMed] [Google Scholar]
- Estimating allowable hemodilution. Anesthesiology. 1974;41:609-12.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of the effects of hydroxyethyl starch on coagulation state of patients during brain tumor surgeries in comparison to crystalloids by thromboelastography. J Res Med Sci. 2014;19:8-12.
- [Google Scholar]
- Effects on coagulation of balanced (130/0.42) and non-balanced (130/0.4) hydroxyethyl starch or gelatin compared with balanced Ringer's solution: an in vitro study using two different viscoelastic coagulation tests ROTEMTM and SONOCLOTTM. Br J Anaesth. 2010;105:273-81.
- [CrossRef] [PubMed] [Google Scholar]
- Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: A prospective randomized trial. Br J Anaesth. 2010;104:691-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical experience of hydroxyethyl starch (10% HES 200/0.5) in cerebral perfusion pressure protocol for severe head injury. Surg Neurol. 2006;66(Suppl 2):S26-31.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-operative hydroxyethyl starch is not associated with post-craniotomy hemorrhage. Springerplus. 2015;4:350.
- [CrossRef] [PubMed] [Google Scholar]
- Flurbiprofen and hypertension but not hydroxyethyl starch are associated with post-craniotomy intracranial haematoma requiring surgery. Br J Anaesth. 2014;113:832-9.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized trial of efficacy of crystalloid and colloid resuscitation on hemodynamic response and lung water following thermal injury. Ann Surg. 1983;197:520-31.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, controlled, double-blind crossover study on the effects of isoeffective and isovolumetric intravenous crystalloid and gelatin on blood volume, and renal and cardiac hemodynamics. Clin Nutr. 2020;39:2070-9.
- [CrossRef] [PubMed] [Google Scholar]
- The brain relaxation and cerebral metabolism in stroke volume variation-directed fluid therapy during supratentorial tumors resection: Crystalloid solution versus colloid solution. J Neurosurg Anesthesiol. 2014;26:320-7.
- [CrossRef] [PubMed] [Google Scholar]
- Definition, evaluation, and management of brain relaxation during craniotomy. Br J Anaesth. 2016;116:759-69.
- [CrossRef] [PubMed] [Google Scholar]
- Safety of acute normovolemic haemodilution with hydroxyethyl starch during intracranial surgery. Neurol India. 2000;48:63-7.
- [Google Scholar]
- Hydroxyethyl starches: Different products--different effects. Anesthesiology. 2009;111:187-202.
- [CrossRef] [PubMed] [Google Scholar]
- The pharmacokinetics and tolerability of an intravenous infusion of the new hydroxyethyl starch 130/0.4 (6%, 500 mL) in mild-to-severe renal impairment. Anesth Analg. 2002;95:544-51.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of hydroxyethyl starch (130 kD) on brain inflammatory response and outcome during normotensive sepsis. Int Immunopharmacol. 2010;10:859-64.
- [CrossRef] [PubMed] [Google Scholar]






