Translate this page into:
Correlation of progesterone receptors and P63 to the histological grade of meningiomas: Review and significance in an African population
*Corresponding author: Onyekachi Itohan Aniume, Department of Morbid Anatomy, College of Medicine, University of Nigeria, Enugu, Nigeria. onyekachi.aniume@unn.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Eluke CC, Aniume OI, Olusina BD, Okafor OC, Olasode BJ. Correlation of progesterone receptors and P63 to the histological grade of meningiomas: Review and significance in an African population. J Neurosci Rural Pract. 2024;15:69-73. doi: 10.25259/JNRP_332_2023
Abstract
Objectives:
Meningiomas, a common neoplasm of the central nervous system, is a widely studied meningeal tumor. According to the World Health Organization (WHO) 2021 classification of meningiomas, there are 15 subtypes that have been grouped into grades 1, 2, and 3. The WHO grade 1 meningiomas are generally grouped as benign while the WHO grades 2 and 3 tumors are grouped as malignant. Progesterone receptors and P63 are common immunohistochemical markers that have proven useful in the diagnosis, grading, and prognostication of many neoplasms such as breast carcinoma, prostate carcinoma, and gastrointestinal tumors in histopathology practice. The application of these immunohistochemical markers to the grading of meningiomas has been reported and their usefulness documented in reports from Africa, Europe, North America, South America, and Asia. This study, therefore, seeks to determine if these findings are applicable to the meningiomas seen in an African population.
Materials and Methods:
A 10-year review of results and histologically diagnosed cases of meningiomas received in the Department of Morbid Anatomy, University of Nigeria, Enugu. Immunostaining for progesterone receptors (PgRs) and P63 were done and results compared with histologic grades.
Results:
The three WHO grades of meningioma were assessed in this study. M: F ratio was 1:1.4 and peak age was 41–50 years age range (SD ± 16.54). The majority of the cases were WHO grade 1 (86.1%) while the WHO grades 2 and 3 tumors were 8% and 5.9%, respectively. The fibrous variant was the most common subtype (27.1%). There was no correlation between progesterone receptor and P63 immunopositivity to the WHO grades of meningioma (P = 0.112 and P = 0.138, respectively).
Conclusion:
Our study showed that progesterone receptors and P63 immunopositivity did not correlate with the WHO grades of meningiomas. This may be due to the predominant variant of meningioma seen in this study. These findings indicate that PgR antagonist may not be an effective alternative for treatment in patients with inoperable meningiomas. Furthermore, P63 immunopositivity may not be a sufficient grading tool for managing meningiomas in our population.
Keywords
Meningioma
Progesterone receptors
P63
Africa
Central nervous system tumors
INTRODUCTION
Meningioma is a commonly diagnosed brain tumor in Nigerian adults.[1,2] They arise from the meningeal arachnoid cells and they exert pressure as a result of growing in enclosed spaces with negligible flexibility. They are mostly benign slow-growing tumors. About 80% of meningiomas are benign. The rest have been classified as atypical (~17%) and anaplastic (~2%) due to their aggressive clinical behavior.[3]
Immunohistochemistry is important in the diagnosis and management of meningiomas. A study by Boulagon-Rombi et al.[4] reported that progesterone receptors showed a decreasing percentage of positivity with increasing the World Health Organization (WHO) grade. P63 immunopositivity in meningiomas has been explored in the grading of meningiomas. Sharifi and Katebi reported 83.2% immunopositivity in the WHO grade 1 tumors and 100% immunopositivity in the WHO grades 2 and 3 tumors.[5]
Progesterone is an endogenous steroid hormone mostly produced by the corpus luteum in the ovaries. The central nervous system expresses PgRs where progesterone exerts regulation of cell function and neuroprotective function.[6] A study comparing progesterone receptor immunostaining in normal meninges and meningioma tissue reported 60.3% and 47.4% positivity, respectively,[7] indicating a reduction in PgRs expression in neoplastic meningeal tissue. Progesterone receptors are transcription factors with two documented isoforms, progesterone receptor A (PRA) and progesterone receptor B (PRB). When unbound to progestins, they are located in the cytoplasm, bound to heat shock proteins. When bound to progestins, they are unbound from the heat shock proteins, undergo structural reconfiguration and nuclear migration.
P63 is a protein product of the transformation-related protein 63 gene in the family of p53 gene. It is a nuclear protein with two known isoforms. The P63 with transactivation domain (TAP63) variant is differentially expressed in the brain.[8] TAP63 has been implicated in heart development and premature aging.[9,10]
Limited data are available on immunostaining of PgRs and P63 in meningiomas in Nigeria. A significant expression of PgRs in different grades of meningiomas varies widely in many studies. This validates the need for further studies of meningiomas in our environment. The evaluation of PgRs and P63 and the correlation to the WHO histological grades of meningiomas in our environment would provide baseline data on the tumor biology, immunohistochemical patterns, and possibility of chemotherapy use for management.
MATERIALS AND METHODS
This study was a 10-year retrospective study of 107 cases of histologically diagnosed meningiomas. One hundred and thirty formalin-fixed paraffin-embedded tissue blocks of meningiomas were reviewed by two pathologists and the WHO grades as defined by the 2021 WHO classification for meningiomas were assigned according to their histological features. The exclusion criteria included cases in which the tissue blocks were missing, damaged, or exhausted and cases with incomplete clinical information.
Immunohistochemical studies with anti-progesterone receptors (PgRs) antibodies (Clone: Monoclonal, Source: Invitrogen, USA, Batch No. PA5-82163) and anti-P63 antibodies (Clone: Polyclonal, prediluted, Source: Invitrogen, USA, Batch No PA5-36069), and Ultra-Sensitive ABC Peroxidase Staining Kit (Source: Thermoscientific™, USA, Batch No. 32052) were used to stain fresh 4 micrometer sections from formalin-fixed paraffin-embedded tissue blocks.
The histologically diagnosed cases of meningioma were classified into the WHO grades based on their morphology on H and E staining. The histological and immunohistochemical aspects of this study were carried out in the histopathology laboratory of the Department of Morbid Anatomy, UNTH, Enugu.
The “2010 ASCO/CAP Guideline Recommendations for Immunohistochemical (IHC) Testing of estrogen receptors and PgR in Breast Cancer” was adapted for interpretation of anti-progesterone and anti-p63 immunostains where brown nuclear staining only was interpreted as positive and blue nuclear staining were interpreted as negative. Progesterone receptors and P63 were interpreted semi-quantitatively using only a percentage of positive tumor cells, no staining indicating absence of positive tumor cells, mild staining indicating 1–10% of positive staining nuclei, moderate staining indicating 11–50% of positive staining nuclei, and intense staining indicating 51–100% of positive staining nuclei.
The data obtained were tabulated using 2016 Microsoft Excel and subsequently analyzed using IBM, SPSS 22 statistical package. Chi-square test was used for the comparison of discontinuous variables with P < 0.05 considered as significant and Kendall’s Tau-b coefficient was used to confirm P-value.
All procedures performed in this study were approved by the University of Nigeria Teaching Hospital IRB (NHREC/05/01/2008B-FWA00002458-IRB00002323; February 12, 2020) in accordance with the 1964 Helsinki Declaration and its later amendments.
RESULTS
One hundred and seven meningiomas met the inclusion criteria in our study. Sixty-two occurred in females and 45 occurred in males giving a female-to-male ratio of 1.4:1. The ages ranged from 6 years to 88 years (SD ± 16.54). The peak age was in the 41–50 years (SD ± 16.54) age group (23.4%) and was closely followed by a 19.6% prevalence in the 51–60 years (SD ± 16.54) age group. Fibrous, meningothelial, and psammomatous meningiomas were the most common variants seen in this study with 27.1% (29 cases), 21.5% (23 cases), and 19.6% (17 cases), respectively.
The overall immunohistochemical staining pattern is presented in Table 1. Progesterone receptor staining was highest in the WHO grade 1 tumors compared to other grades while P63 was highest in WHO grade 2 tumors, also reported in Table 1. The statistical relationship between the WHO grade and the immune stains is shown in Table 2. The PgR and P63 staining is shown in Figures 1 and 2], respectively.
| Variables | Grade 1 (n=94) | Grade 2 (n=8) | Grade 3 (n=5) |
|---|---|---|---|
| n(%) | n(%) | n(%) | |
| Progesterone receptor | |||
| No staining | 57(60.6) | 6(75.0) | 5(100.0) |
| Mild | 20(21.3) | 1(12.5) | 0(0.0) |
| Moderate | 16(17.0) | 0(0.0) | 0(0.0) |
| Intense | 1(1.1) | 1(12.5) | 0(0.0) |
| (P=0.112) | |||
| P63 | |||
| No staining | 68(72.3) | 3(37.5) | 3(60.0) |
| Mild | 13(13.8) | 2(25.0) | 0(0.0) |
| Moderate | 7(7.4) | 1(12.5) | 0(0.0) |
| Intense | 6(6.4) | 2(25.0) | 2(40.0) |
| (P=0.138) | |||
| Diagnosis/Type | Kendall’s Tau-b | P-value |
|---|---|---|
| Grade | 0.118 | 0.112 |
| PR | ||
| Grade | 0.195 | 0.138 |
| P63 |
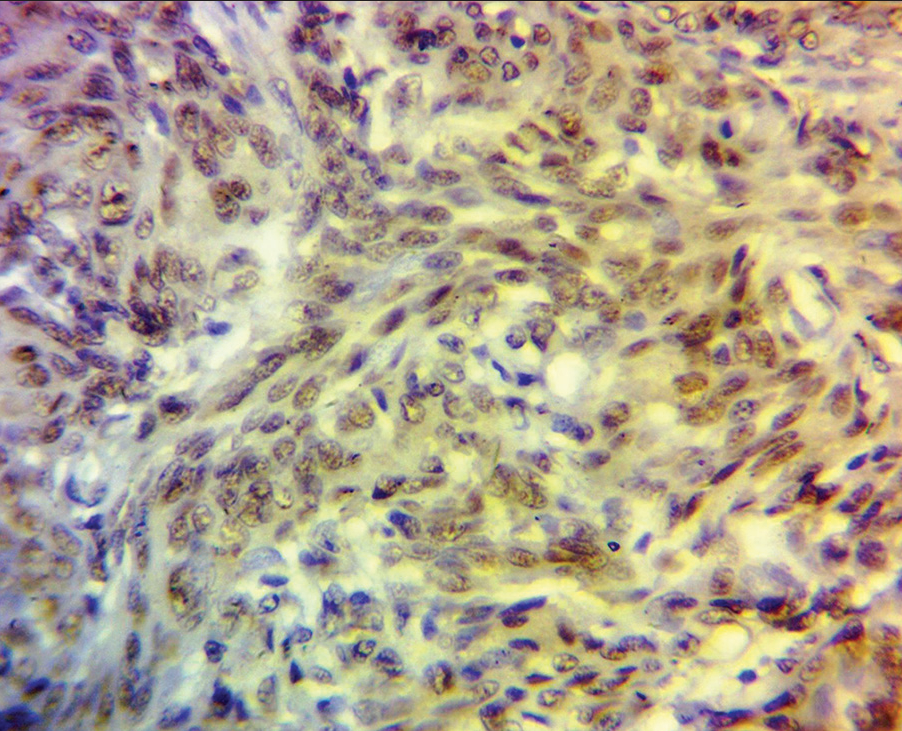
- Photomicrograph shows intense progesterone receptor immunopositivity in a World Health Organization grade 1 meningothelial meningioma.
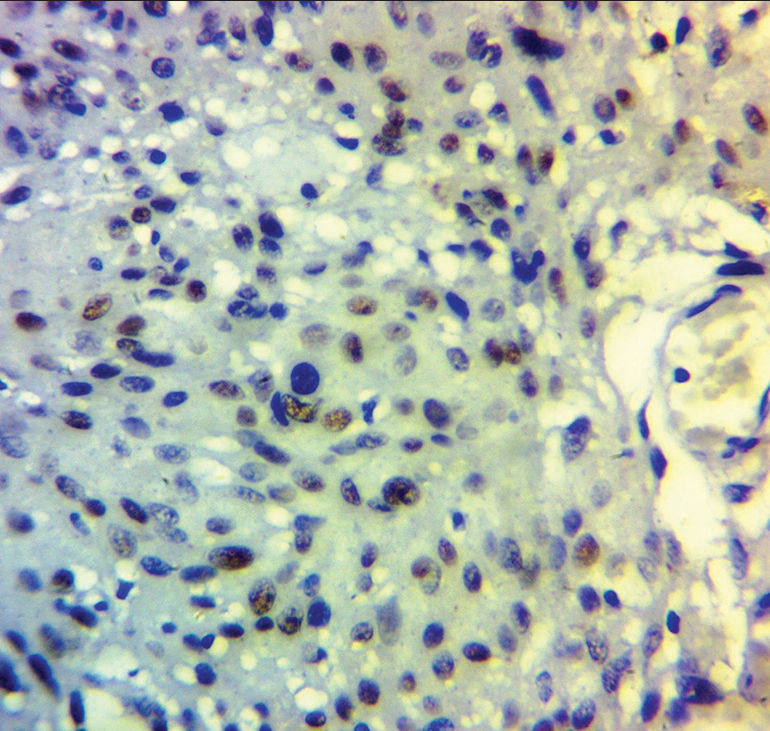
- Photomicrograph shows P63 immunopositivity in the World Health Organization grade 2 atypical meningioma.
DISCUSSION
For the immunostains, progesterone receptors and P63 showed an average of 45.5% and 30.8% positivity, respectively, across the three WHO grades that were studied. The immunopositivity of progesterone receptors showed an inverse correlation to the WHO grades; grade 1 (39.4%), grade 2 (25%), and grade 3 (0%). Progesterone is an endogenous steroid hormone involved in reproduction and embryogenesis. It also plays an important role as a neurosteroid where it interacts with non-nuclear PgRs and sigma receptors to act as neuromodulators. In this study, there was no statistical significance between PgR immunostaining and the WHO grades of meningioma. The reduced progesterone receptor immunoreactivity in this study may be due to the prevalent type of the WHO grade 1 meningioma found in this study (fibrous variant). This finding is partly supported by the study done by Omulecka et al.[11] where 64 meningioma samples were studied with 21 transitional and 13 fibrous variants. They reported a 42.2% immunopositivity in fibrous meningioma as against 100% immunopositivity in meningothelial meningioma. There are varying reports on PgR immunoreactivity and the WHO grades of meningioma in the literature. Ikeri et al.,[12] in their study in Lagos, Nigeria, reviewed 72 meningioma cases in their study and they found an inverse correlation between the WHO grades and the progesterone receptor expression. However, more cases were investigated in our study and the common subtypes were at variance with the findings from Lagos, Nigeria. Csonka et al.[13] and Wolfsberger et al.[14] both found an inverse relationship between the WHO grade of meningioma and PgR positivity in their studies, PerrotApplanat et al.[15] and Ceylan and Ozmen.[16] both reported a weak inverse correlation. A systematic review by Agopiantz et al.[17] showed that 72.2% of meningiomas expressed PgRs; however, the majority of the articles that were reviewed were not studies done on the indigenous African population, thus raising the possibility of varying expression of PgR expression in the meningiomas seen in the African population.
P63 immunoreactivity showed positive staining in 27.6% of the WHO grade 1 tumors, 62.5% of the WHO grade 2 tumors, and 40% of the WHO grade 3 tumors. This was not statistically significant (P = 0.138). Reports from studies conducted in Iran, India, and USA have shown increasing immunopositivity of P63 with increasing WHO grades of meningiomas.[5,18,19] In this study; however, a significant percentage of the WHO grade 1 tumors showed immunopositivity (27.6%) similar to the report by Mittal et al.[20] in India where their study showed 34.9% immunopositivity in the WHO grade 1 tumors thus concluding that it cannot be used as a sole marker in grading meningiomas.
In our study, we found a poor correlation between PgRs and P63 with the WHO grade of meningioma. Due to the poor expression of progesterone receptors, the use of anti-progestins as an alternative chemotherapeutic agent may not be beneficial to patients in our environment.
CONCLUSION
Contrary to many other study findings, this study did not show any correlation between progesterone receptors and P63 with the WHO grades of meningioma. This may be due to varying degree of expression of progesterone receptors and P63 in different variants. Thus, we concluded in this study that progesterone receptors and P63 immunostains may not be beneficial for grading meningiomas in cases submitted for histopathological assessment.
Acknowledgment
Prof. Martin A. Nzegwu for the support during the study.
Ethical approval
All procedures performed in this study were approved by the University of Nigeria Teaching Hospital IRB (NHREC/05/01/2008B-FWA00002458-IRB00002323; February 12, 2020) in accordance with the 1964 Helsinki Declaration and its later amendments.
Declaration of patient consent
The Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Histo-pathological pattern of intracranial tumours in the National Hospital, Abuja. Afr Health Sci. 2018;18:281-6.
- [CrossRef] [PubMed] [Google Scholar]
- Association of race with survival in intracranial world health organization Grade II and III meningioma in the United States: Systematic literature review. World Neurosurg. 2020;138:e361-9.
- [CrossRef] [PubMed] [Google Scholar]
- CBTRUS Statistical Report: Primary brain and other central nervous system tumours diagnosed in the United States in 2010-2014. Neurooncol Adv. 2017;19:v1-88.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical approach to the differential diagnosis of meningiomas and their mimics. J Neuropathol Exp Neurol. 2017;76:289-98.
- [CrossRef] [PubMed] [Google Scholar]
- An immunohistochemical study of p63 protein expression in meningioma. Iran J Pathol. 2008;3:146-50.
- [Google Scholar]
- Progesterone receptors: Form and function in brain. Front Neuroendocrinol. 2008;29:313-39.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical expression of aromatase and estrogen, androgen and progesterone receptors in normal and neoplastic human meningeal cells. Neuropathology. 2010;30:44-9.
- [CrossRef] [PubMed] [Google Scholar]
- TP63 (Tumor protein P63) Atlas of genetics and cytogenetics in oncology and haematology; 2012 Available from: https://www.atlasgeneticsoncology.org/gene/365/tp63-(tumour-protein-p63) [Last accessed on 2021 Mar 04]
- [CrossRef] [Google Scholar]
- TAp63 is important for cardiac differentiation of embryonic stem cells and heart development. Stem Cells. 2011;29:1672-83.
- [CrossRef] [PubMed] [Google Scholar]
- TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell. 2009;5:64-75.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical expression of pregesterone and estrogen receptors in meningiomas. Folia Neuropathol. 2006;44:111-5.
- [Google Scholar]
- Progesterone receptor expression and Ki-67 labelling index of meningiomas in the Lagos university teaching hospital. Niger Postgrad Med J. 2018;25:17-20.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of candidate immunohistochemical prognostic markers of meningioma recurrence. Folia Neuropathol. 2016;54:114-26.
- [CrossRef] [PubMed] [Google Scholar]
- Progesterone-receptor index in meningiomas: Correlation with clinico-pathological parameters and review of the literature. Neurosurg Rev. 2004;27:238-45.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical study of progesterone receptor in human meningioma. Acta Neurochir (Wien). 1992;115:20-30.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic importance of progesterone receptor expression in meningioma. Ann Med Res. 2020;27:110-5.
- [CrossRef] [Google Scholar]
- Hormone receptor expression in meningiomas: A systematic review. Cancers (Basel). 2023;15:980.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic significance of Ki-67 labelling index and p63 immunoreactivity in intracranial and intraspinal meningiomas. Ann Pathol Lab Med. 2016;3:458-64.
- [Google Scholar]
- Correlation of p63 immunoreactivity with tumor grade in meningiomas. Int J Surg Pathol. 2008;16:38-42.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation of p63 protein expression with histological grade of meningiomas: An immunohistochemical study. Int J Surg Pathol. 2012;20:349-54.
- [CrossRef] [PubMed] [Google Scholar]







