Translate this page into:
Corpus Callosum and Neglect Syndrome: Clinical Findings After Meningioma Removal and Anatomical Review
Address for correspondence: Dr. José Pedro Lavrador Rua Cidade de Faro N° 40 2725-689 Mem-Martins, Lisboa Portugal E-mail: jose.pedro.lavrador@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Two types of neglect are described: hemispatial and motivational neglect syndromes. Neglect syndrome is a neurophysiologic condition characterized by a malfunction in one hemisphere of the brain, resulting in contralateral hemispatial neglect in the absence of sensory loss and the right parietal lobe lesion being the most common anatomical site leading to it. In motivational neglect, the less emotional input is considered from the neglected side where anterior cingulate cortex harbors the most frequent lesions. Nevertheless, there are reports of injuries in the corpus callosum (CC) causing hemispatial neglect syndrome, particularly located in the splenium. It is essential for a neurosurgeon to recognize this clinical syndrome as it can be either a primary manifestation of neurosurgical pathology (tumor, vascular lesion) or as a postoperative iatrogenic clinical finding. The authors report a postoperative hemispatial neglect syndrome after a falcotentorial meningioma removal that recovered 10 months after surgery and performs a clinical, anatomical, and histological review centered in CC as key agent in neglect syndrome.
Keywords
Corpus callosum
meningioma
neglect
INTRODUCTION
The right hemisphere is the dominant for the visuospatial capacities (spatial perception and spatial memory): recognition of the surrounding space, complex visual stimuli (such as a human face), and selective hemispatial attention. These capacities are performed by a neural network that includes the temporal-parietal junction, the inferior frontal gyrus, and inferior parietal lobule (IPL) of the right hemisphere.[1]
Neglect is clinically defined as the impaired or loss of ability to react to or process sensory stimuli when presented in the hemispace contralateral to a brain lesion in the absence of any remarkable sensory lost.[1] These patients will bump into objects or obstacles on their left side; if asked to draw an object, they will draw only the right side, they may read only the right pages of a book, and they may neglect the left side of their body.[2]
There are different types of neglect: visual, spatial (sometimes called visuospatial neglect), and motivational neglect. It is difficult to dissociate visual neglect from spatial neglect because one needs visual input to have a proper and accurate notion of space. This visual input will be associated with sensory input (proprioceptive sensibility) in the IPL. However, the IPL of the right hemisphere can integrate visual and proprioceptive information from both hemispaces, and the left one does the same only for the right hemispace.[3]
There are two hypotheses concerning the pathophysiology of neglect. One supports the hemispheric specialization in which the left hemisphere controls the orientation of attention for the right visual half-field, whereas the right hemisphere controls the orientation of attention for both visual half-fields. This theory is supported by the prevalence of neglect in the right hemisphere injuries and by imaging studies that demonstrate a predominant activation of the right hemisphere over the left one during actions that involve shifts in visuospatial attention.[4] Besides patients with an injury on the left hemisphere do not usually develop right hemispace neglect. The other theory, hemisphere competition hypothesis (Kinsbourne's theory), supports the existence of a dynamic asymmetric balance between the frontoparietal circuits of both hemispheres, with the right one prevailing.[4] Damage of the white matter fiber bundles connecting the parietal to the frontal lobe is a relevant pathophysiological component of the neglect syndrome.[5]
Thus, since the right hemisphere can orient attention for both sides,[1] if there is a loss of function in the left hemisphere, the right hemisphere is able to compensate for that.[2] This is possible due to the passage of connecting fibers from the left hemisphere to the right one through the corpus callosum (CC).[1] However, if there is a damage in the right hemisphere, the left hemisphere is not able to compensate the loss[2] because the left hemisphere can only orient attention for the right hemispace.[1] Thus, a lesion on the right hemisphere can lead to left neglect whereas a lesion on the left hemisphere rarely leads to right neglect.[1] Nevertheless, it is important to realize that there are still questions about the basis of neglect syndrome.[6]
The principal structure of the mammalian brain that connects the two cortical hemispheres is the CC.[4] Structural defects in the CC during development often result in significant neuropsychological dysfunction. The CC is responsible for interconnecting homologous and heterologous cortical association areas of both hemispheres[4] and it is associated with the transmission of cognitive, somatosensory, motor, executive, and visual information.[78] With approximated 180 million callosal fibers passing through it, the CC receives abundant blood supply from both the anterior and posterior cerebral circulation.[4] Besides the CC, there are also two more structures that interconnect the two hemispheres: The anterior commissure (that interconnects the olfactory system and parts of the limbic system) and the hippocampal or posterior commissure (that interconnects parts of the limbic system); however, the CC is the largest fiber tract in the human brain.[910]
The CC is constituted by three types of fibres: forceps minor, transversal fibers, and forceps major. The forceps minor has fibers that travel from the genu of CC to the frontal lobes, connecting them both. The forceps major irradiates from the splenium of CC to occipital lobes. The transversal fibers or radiation of CC will extend laterally and intersect association fibers and projection fibers that pass through multiple cortex areas.[11]
Anatomically, the CC can be divided into four parts from the posterior to anterior: splenium, body, genu, and rostrum.[1213]
The splenium connects occipital, posterior parietal, and middle temporal cortices with the correspondent areas of the contralateral hemisphere.[12] These areas are connected through fibers that differ in their size and other histological characteristics and therefore are called heterogeneous. Some fibers are reciprocal and connect the hemispheres in a homotypical manner, but there are also fibers that connect the same areas, but in a heterotypical manner. The physiological role of the CC is thought to be either excitatory or inhibitory. The excitation refers to the tendency of one cortical area to activate the symmetrical area of the contralateral hemisphere, whereas in inhibition the opposite occurs. Because the majority of long-distance cortico-cortical connections are excitatory, the suppression induced from one hemisphere to the other one must include local inhibitory interneurons. Therefore, the somation of the interhemispheric effects results from diversified events at the neuronal level; events that occur in the splenium of CC.[14]
The fibers of the anterior portion of the splenium were myelinated later in the development process, so they are thin and mostly responsible for connecting the middle and inferior temporal and parietal association areas. This slow myelinization correlates to excitatory influences between the hemispheres. On the other hand, the fibers in the posterior portion of the splenium that connect the primary and secondary visual areas in the V1 (primary visual area)/V2 (secondary visual area) border (calcarine sulcus) were myelinated earlier than the anterior fibers of the splenium, so they are thicker. The gradual increment in inhibitory effects during the development in the striated cortex is thus related to local inhibitory circuits such as inhibitory interneurons.[14]
New studies have questioned some of the previous notions about the splenium of the CC and its role, such as the assumed symmetry between callosal connections. It was shown that there is a higher interhemispheric connection from the right hemisphere to the left one, in the extrastriate cortices.[14]
The major sources of blood flow to the CC are the internal carotid artery and the vertebrobasilar system.[15] The anterior cerebral artery (ACA), a branch of the internal carotid artery, sends the pericallosal artery, which is located in the superior surface of the CC, traveling from anterior to posterior in this convex surface. A median callous artery, a branch of the anterior communicating artery, occurs in 20%–80% of the cases and irrigates the genu of the CC.[16] Sometimes, a terminal branch of the pericallosal artery from the ACA supplies the contralateral splenium of CC.[17] The splenium of the CC is irrigated by the vertebrobasilar system or the posterior pericallosal artery in some cases. This artery can be a branch of the P3 segment of the posterior cerebral artery (PCA) or the parietal-occipital artery, the terminal branch of the PCA. The splenial artery circumvents the splenium to anastomose with the anterior pericallosal artery anteriorly at the level of the splenium. Thus, a watershed territory is formed between the irrigation territory of the ACA and of the PCA.[5] The perforating arteries branching from these two systems are responsible for the irrigation of the CC, and this arterial system surrounds all of its constituting fibers.[15]
Lesions in the splenium of corpus callosum
The injuries to CC can have several etiologies, such as gliomas, lymphomas, infections, desmielinizated plates, associated, for example, with multiple sclerosis and although less frequently, ischemic lesions (rich collateral circulation) and iatrogenic lesions after surgical approaches (infratentorial supracerebellar approach, posterior interhemispheric approach, and its variants among others).[1819]
CASE REPORT
A 63-year-old patient, with significant past medical history of large B-cell lymphoma treated with methotrexate-base chemotherapy and radiotherapy 17 years before, was admitted to the emergency department for progressive impaired consciousness state. A computed tomography and a magnetic resonance imaging (MRI) head were done revealing the right falcotentorial space occupying lesion with surrounding vasogenic edema. The patient had a right side parieto-occipital craniotomy and tumor removal through the right posterior interhemispheric approach (left lateral decubitus). The neuropathology examination revealed a WHO Grade 1 meningioma (meningothelial). Postoperatively, the patient showed inattention to the left side, anosognosia, hypoarousal, motor intentional deficits (left side hemiparesis), and left side apraxia. A postoperative MRI shows as an ischemic lesion in the splenium of the CC [Figure 1]. The patient was started in a motor rehabilitation program. She was bedridden at hospital discharge, in a wheelchair at 6 moths postoperative, and walking with support at 10 months postoperative with partial recovery from the neglect syndrome presented after surgery.
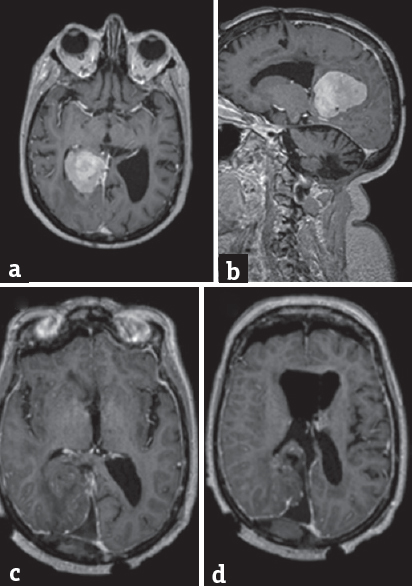
- (a) (Axial T1Gad preoperative), (b) (sagittal T1Gad preoperative), (c and d) (axial T1Gad postoperative) – right falcotentorial meningioma completly ressected. Ischemia of corpus callosum showed by the hypointensity in the splenium (arrow)
Anatomy of neglect syndrome
Neglect can be caused by lesions in multiple anatomical locations.[20] The temporo-occipital junction, the inferior, middle, and superior temporal gyrus, and the IPL are demonstrated to be important to respond to stimulus of the left hemispace.[21] Thus, lesions in these sites are related to hemispatial neglect. For example, since the IPL is involved in sensorimotor representation of extrinsic situations, it is clear that a lesion in parietal cortex leads to contralateral hemineglect and hemi-inattention. Disconnection in uncinate bundle, frontoparietal and frontotemporal segments of arcuate bundle and cortico-ponto-cerebelar tract can also lead to neglect.[20]
A combined injury of the left occipital lobe and the splenium of CC or forceps major can lead to visual hemispace neglect due to the inability of visual information to gain access to the contralateral occipital lobe that processes this information. The posterior splenium may transfer nonverbal visual information, whereas the anterior splenium may transfer verbal-visual information.[21] A disconnection syndrome in the splenium does not affect the auditory and tactile pathways because these sensory modalities are transferred more anteriorly than the splenium of CC.
It is important to refer that a lesion of lateral dorsal nucleus, lateral posterior nucleus, and anterior nucleus of thalamus also can lead to spatial neglect. These nucleus receive inputs from superior colliculus and pretectum nucleus (mesencephalic visuomotor complex) and from premotor and primary motor cortices. The thalamus sends outputs to superior and IPLs. These structures have reciprocal connections with association areas and allow cortico-cortical communications, modulating functions that need visual-sensory-motor integration. A lesion in the thalamus can lead to an indirect frontoparietal disconnection, which leads to spatial neglect.[20]
The posterior temporoparietal and the frontal areas are also known to be the anatomical areas of hemispatial neglect. Neglect may be induced by perception-attention and attention-action abnormalities. The temporoparietal area is responsible for the perception-attention and the frontal area for the attention-action mechanisms.[22]
When a lesion involves the superior longitudinal fasciculus (SLF), like an infarction of the medial cerebral artery (MCA), it will cause a disconnection syndrome, because SLF contains frontoparietal and fronto-occipital bundles that allow visual information to be used for total perception of contralateral hemispace and hemibody. Infarction of MCA can also damage fronto-temporal fibers of the SLF.[20]
It is known that hemispatial neglect most frequently results from cortical-subcortical lesions in the territory of the MCA and also from lesions affecting basal ganglia. Visual neglect results from lesions in the splenium, mainly in the territory of PCA, and it is usually accompanied by hemianopsia.[5]
Types of neglect
Visuospatial neglect
In Figures 2 and 3, one can see the difference of spatial mapping between a healthy individual and an individual with left visual neglect. In a healthy person, the right parietal lobe receives visual information from both ocular fields and sensory information from the left side of the body, whereas the left parietal lobe receives visual information only from the right ocular field and sensory information from the right side of the body. Since parietal lobes spatially map this information, the mind can spatially locate stimuli. In an individual with Crigler-Najjar syndrome (damage of the right parietal lobe), parietal lobes also receive visual and sensory information; however, the intact left parietal lobe can spatially locate stimuli (only from the right side) while the right parietal lobe is not. Thus, the information from the left ocular field and from the left part of the body never becomes a part of the conscious awareness resulting in the inability to see or feel stimuli from this side[2] [Figure 4].
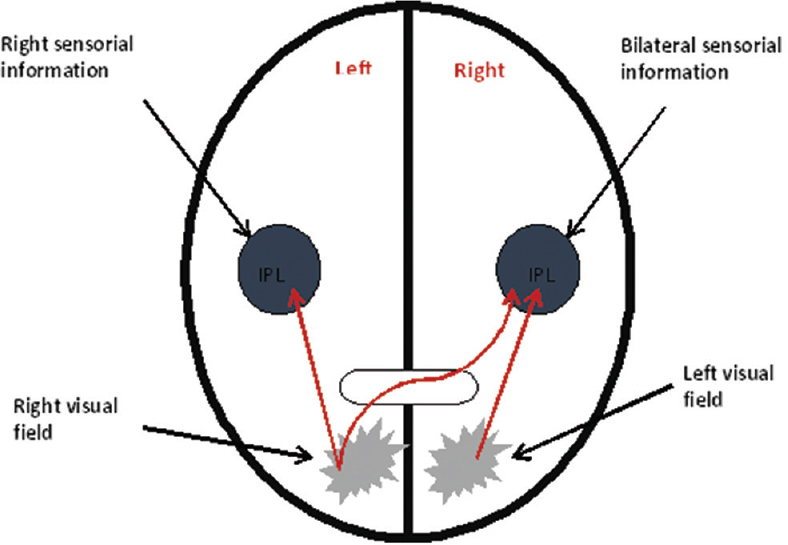
- Integration of visual and sensitive information. IPL: Inferior parietal lobe
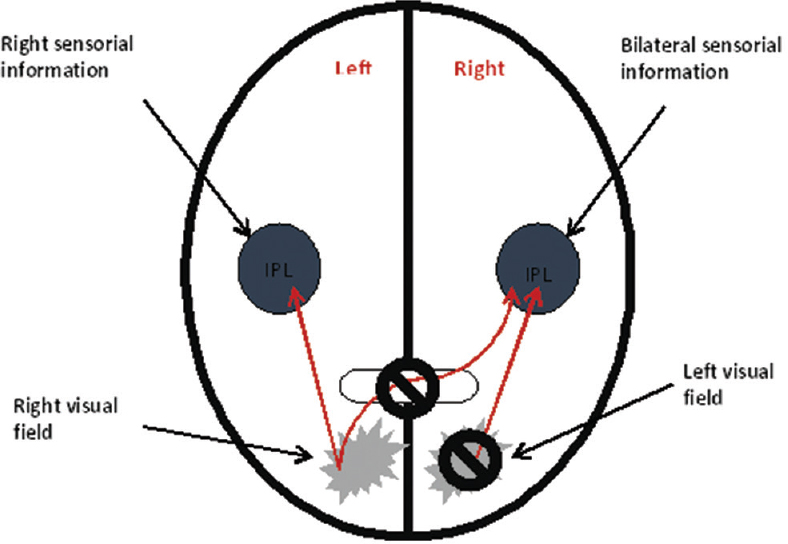
- Left visual neglect. IPL: Inferior parietal lobe
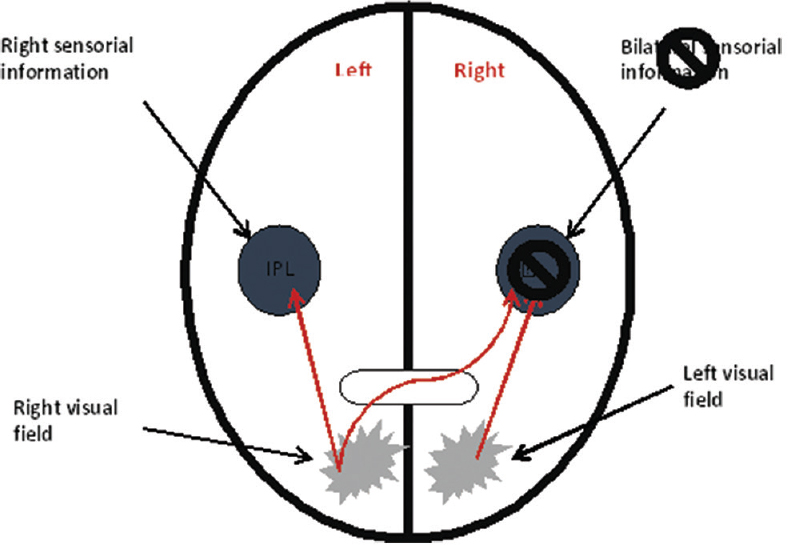
- Hemispatial neglect
The visual and nonvisual sensory space processing pathways start when ganglion cells of the retina capture visual stimuli. The visual information passes through the optic nerve and goes to the lateral geniculate body and other areas in the thalamus, to the occipital lobe, to the parietal lobe and then back to the thalamus. The information proceeds to the frontal lobe and prefrontal cortex to be further processed and then goes back to the thalamus, allowing this information to become conscious. A spatial/sensory input from the body (afferent pathway) is involved in feedback loops between the thalamus and the parietal/frontal lobes. The thalamus is also important for the integration of this sensory information and allows the formation of a three-dimensional (3D) image of the visual field, through his connections with parietal and frontal lobes.[2]
Left visual half-field goes from the right hemisphere to the left parieto-frontal area to be processed in a 3D manner, along with other sensory-motor information from the surroundings (such as proprioceptive information about the position of the different parts of the body), provided by other pathways [Figure 3]. A lesion in splenium of CC prevents this passage of information so that the patient will neglect this part of the body and space – pure alexia without agraphia. When commissural fibers of CC that carry right visual information for language areas in the left hemisphere (Wernicke's area and Broca's area) are injured, the patient will have difficulty in reading, although visual and verbal function are preserved. This means that the words of left visual half-field will not be processed and integrated in Wernicke's temporal area, and the patient will be presented with agraphia – pure disconnection syndrome.[20] Jerath and Crawford proposed that this visual information is lost when it is processed or attempted to be processed, by the damaged parietal lobe. The parietal lobe is not able to spatially map the visual information, so when this information is sent back to the thalamus via feedback loops, it is not projected within the 3D default space and therefore does not rise to conscious awareness.[2]
The left temporo-occipital junction contains the visual word form area, which processes the visual component of reading. A disruption in this area or in its input/output can lead to the left hemialexia (the patient ignores the first letter of each word). Words of the left visual field project to the right visual area in occipital lobe; then, through the splenium of the CC, this information reaches the left fusiform cortex. Therefore, an injury in the splenium of the CC leads to alexia without agraphia because writing does not depend on visual input for the language areas.[23] This inability to read in the left visual half-field may be accompanied by the so – called color anomia (patients are not able to name the colors presented, although they can name the color of an object without seeing it).[24] When the hemialexia and associated deficits are most severe, they can be mistaken for a left homonymous hemianopsia. Hemialexia can be considered to be of two types: An inability to match written words with objects and an inability to read aloud written words or letters. Both of these are said to be mimicked by the condition of visual hemineglect.
Concerning the recognition of objects, if one places an object in the right hand of a patient with a dominant left hemisphere with closed eyes, he will be able to recognize and describe the object by touch. This recognition does not happen if the object is placed in the left hand of the patient because the tactile information travels to the right postcentral gyrus but is not capable of passing through CC to the left dominant hemisphere and reach Broca's area of motor speech. Therefore, even though recognizing the object, the patient is not capable of describing it. This symptom is called left tactile anomia.[11]
Additional dysfunctions in the following characteristics that may be found in individuals with spatial neglect, such as: (1) Self-monitoring (unawareness of their deficit – anosognosia – or unconcerned about it – anosodiaphoria), (2) emotional processing (difficulty in making appropriate emotional facial expressions and may lack normal affect or vocal intonation, representing emotional knowledge or understanding emotional information presented via others vocal prosody or facial expressions), (3) arousal (hypoarousal primarily), (4) motor intentional deficits (motor neglect and premotor neglect, impairment in activating or directing actions into portions of space), (5) personal neglect (unable to attend to the left side of their body).[25]
Three phases have been described in neglect syndrome: Acute (lasting a few weeks), subacute (lasting <3 months), and chronic (persistent neglect, lasting more than a year). The first two are more common after mild strokes and are reversible.[26] The persistent neglect is more common in the elderly, in patients with demented and atrophic brains, in patients with anosognosia or hemianopsia, and also in patients who suffer a severe acute phase. A third of the patients who suffered a stroke in splenium of CC stays in a chronic phase.[5]
Motivational neglect
The cingulate cortex of limbic system integrates motivational processes of extrinsic events. The anterior cingulate cortex (ACC) has the ability to process motivational and reward events and associate them with sensory-motor actions. Through the cingulate cortex, there is a communication between different cortical and subcortical structures with different functions. A lesion in the ACC can provoke a form of neglect: Motivational neglect. Patients will behave as if the neglected hemispace is motivationally diminished. Lesion of unilateral and selective ACC leads to learning deficits based on motivational reward.[27] This can also happen in a lesion in parieto-frontal areas and connections (through SLF), IPL, and in opercular cortex. There are studies that show that, if there is enough motivation (like a reward), a person can improve from this condition. The mesolimbic pathway may be involved in this process.[27]
Corpus callosum and neglect: Is it the end of the story?
Even though patients with dysgenesis of CC remain free of the most disabling features of a disconnection syndrome, a single lesion in CC after its formation is sufficient to produce this syndrome. This was first realized by the Nobel Prize laureate Sperry in 1968[28] and since then multiple theories have tried to explain this finding. On the other hand, the inhere reported case, as well as other reports in the literature,[293031] supports the possible recovery potential of the neglect and disconnection syndromes associated with CC lesions.
The previous described situations support the role played by neuroplasticity in the relationship between CC and neglect syndrome. The existence of aberrant white matter connections in the same hemisphere or between both hemispheres (Probst bundles, asymmetric sigmoid bundle, interhemispheric midbrain bundle and interhemispheric ventral forebrain bundle)[32] supports the dynamic compensation that exits early in development that may overcome the CC function. On the other hand, the partial recovery seen in most of the patients (mainly from traumatic etiology),[3031] may indicate some CC functions have widespread representation while others are more location-specific and less prone to recover after being affected by a lesion.
CONCLUSION
The CC is essential in sensory experience since it transfers information from one hemisphere to another. In a CC dysfunction, each hemisphere becomes isolated, acting as two separate brains. Although general intelligence and behavior appear normal, other functions are affected. Even though the parietal lobe is the one classical related with the neglect syndrome, other structures are involved in its anatomy, as the CC. In neglect, there is lack of a complete cognitive interpretation and integration with other sensitive and sensorial modalities, leading to a lack of awareness of the side contralateral to the hemispheric lesion. Neurosurgical pathology, as tumors of vascular injuries, as well as postoperative clinical pictures due to neurosurgical approaches may manifest with a neglect syndrome that should be recognized by the neurosurgeon.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Selective reorienting response of the left hemisphere to invalid visual targets in the right side of space: Relevance for the spatial neglect syndrome. Cortex. 2015;65:31-5.
- [Google Scholar]
- Neural correlates of visuospatial consciousness in 3D default space: Insights from contralateral neglect syndrome. Conscious Cogn. 2014;28:81-93.
- [Google Scholar]
- Microstructural damage of the posterior corpus callosum contributes to the clinical severity of neglect. PLoS One. 2012;7:e48079.
- [Google Scholar]
- Selective visual neglect in right brain damaged patients with splenial interhemispheric disconnection. Exp Brain Res. 2010;206:209-17.
- [Google Scholar]
- Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17:2479-90.
- [Google Scholar]
- Callosal disconnection syndrome after corpus callosum infarct: A diffusion tensor tractography study. J Stroke Cerebrovasc Dis. 2013;22:e240-4.
- [Google Scholar]
- MRI-defined corpus callosal atrophy in multiple sclerosis: A comparison of volumetric measurements, corpus callosum area and index. J Neuroimaging. 2015;25:996-1001.
- [Google Scholar]
- Contralateral targeting of the corpus callosum in normal and pathological brain function. Trends Neurosci. 2015;38:264-72.
- [Google Scholar]
- Alien hand, restless brain: Salience network and interhemispheric connectivity disruption parallel emergence and extinction of diagonistic dyspraxia. Front Hum Neurosci. 2016;10:307.
- [Google Scholar]
- Attention and spatial cognition: Neural and anatomical substrates of visual neglect. Ann Phys Rehabil Med 2016 pii: S1877-065700017-8
- [Google Scholar]
- The corpus callosum, the other great forebrain commissures, and the septum pellucidum: Anatomy, development, and malformation. Neuroradiology. 2010;52:447-77.
- [Google Scholar]
- Splenium of corpus callosum: Patterns of interhemispheric interaction in children and adults. Neural Plast 2013 2013:639430.
- [Google Scholar]
- Callosal disconnection syndrome in a patient with corpus callosum hemorrhage: A diffusion tensor tractography study. Arch Neurol. 2012;69:1374-5.
- [Google Scholar]
- Arterial and venous vascularization of the corpus callosum. Neurochirurgie. 1998;44(1 Suppl):31-7.
- [Google Scholar]
- Surgical management of anterior communicating and anterior cerebral artery aneurysms. In: Schmidek and Sweet Operative Neurosurgical Techniques Indications, Methods, and Results (6th ed). Philadelphia: Elsevier/Saunders; 2012.
- [Google Scholar]
- Callosal disconnection syndrome in a left-handed patient due to infarction of the total length of the corpus callosum. Neuropsychologia. 1999;37:253-65.
- [Google Scholar]
- Infarction of the corpus callosum: A retrospective clinical investigation. PLoS One. 2015;10:e0120409.
- [Google Scholar]
- Unilateral spatial neglect in degenerative brain pathology. Neuropsychology. 2011;25:554-66.
- [Google Scholar]
- Damage to white matter pathways in subacute and chronic spatial neglect: A group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cereb Cortex. 2014;24:691-706.
- [Google Scholar]
- Clinical Neuropsychology (5th ed). New York: Oxford University Press; 2012.
- Left hemispatial visual neglect associated with a combined right occipital and splenial lesion: Another disconnection syndrome. Neurocase. 2005;11:310-8.
- [Google Scholar]
- Left hemiparalexia of Chinese characters: Neglect dyslexia or disruption of pathway of visual word form processing? Brain Struct Funct. 2014;219:283-92.
- [Google Scholar]
- Neuroanatomy of hemispatial neglect and its functional components: A study using voxel-based lesion-symptom mapping. Brain. 2010;133(Pt 3):880-94.
- [Google Scholar]
- White matter lesional predictors of chronic visual neglect: A longitudinal study. Brain. 2015;138(Pt 3):746-60.
- [Google Scholar]
- Cingulate neglect in humans: Disruption of contralesional reward learning in right brain damage. Cortex. 2015;62:73-88.
- [Google Scholar]
- Non-surgical treatment of massive traumatic corpus callosum hematoma after blunt head injury: A case report. Neurol Neurochir Pol. 2016;50:309-12.
- [Google Scholar]
- Partial recovery from visual object agnosia: A 10 year follow-up study. Cortex. 1993;29:529-42.
- [Google Scholar]
- Temporary and permanent signs of interhemispheric disconnection after traumatic brain injury. Neuropsychologia. 2003;41:634-43.
- [Google Scholar]
- Structural and functional brain rewiring clarifies preserved interhemispheric transfer in humans born without the corpus callosum. Proc Natl Acad Sci U S A. 2014;111:7843-8.
- [Google Scholar]






