Translate this page into:
Complex Neurovascular Syndromes: Is the Compressing Vessel Alone the Culprit?
Aniruddh Kulkarni, MCh Department of Neuro and Spine surgery, Neuro World and Suchirayu Hospital Hubli 580029, Karnataka India draniruddh@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective To describe and correlate the clinical, radiological, and intraoperative findings in patients with refractory neurovascular syndromes (NVS) not responding to conventional medical management and to determine the surgical outcome of the microvascular decompression (MVD) procedure.
Methods Medical records of 17 patients with NVS (trigeminal neuralgia [TN] = 14 and hemifacial spasm = 3) who underwent surgery for symptom relief from January 2018 to July 2021 with follow-up data (1–36 months) were retrospectively analyzed. Patient demographics (age, sex), clinical features (site, duration of symptoms, distribution), magnetic resonance imaging (MRI) findings, micro-neurosurgical details (type of surgery, obstructive vessel), and postoperative outcome and complications were recorded.
Statistical Analysis Descriptive analysis was performed. Variables were presented as either mean and standard deviation or frequency and percentages.
Results The mean (standard deviation) age of patients in our study cohort was 52.6 (12.2) years. TN was common in females (64.3%). The mean duration of symptoms was longer in patients with hemifacial spasms than in patients with TN (3.3 vs. 2.7 years). While the right side was commonly affected in TN (64.3%), the left side was common in hemifacial spasm (66.7%). Most common neuralgia symptoms were distributed along the V2V3 (maxillary and mandibular division) branches (42.9%). MRI revealed neurovascular conflict in nine patients, epidermoid tumor in three patients, classical vestibular schwannoma in two patients, and short cisternal segments in three patients. Intraoperatively, superior cerebellar artery was the main offending vessel in TN followed by anterior inferior cerebellar artery (AICA) and venous compression, while tortuous vertebral artery and AICA along with thickened entangled arachnoid were seen in hemifacial spasms. Almost all patients (88.2%) reported immediate postoperative complete pain relief. One patient died secondary to chest infection after a month.
Conclusion Arachnoid entanglement around the neurovascular bundle along with vascular compression over the cranial nerves is the main cause of NVS. Advanced micro-neurosurgical techniques used in MVD achieve excellent outcomes with improved quality of life. However, identifying the refractory NVS not responding to conventional medical management and early surgical management are paramount.
Keywords
neurovascular syndromes
trigeminal neuralgia
hemifacial spasm
microvascular decompression
arachnoid entanglement
Introduction
Neurovascular syndromes (NVSs) are characterized by cranial neuropathy secondary to any irritating lesion, mostly by a vascular loop. The condition is primarily caused by an aberrant or tortuous vessel causing either irritation or direct mechanical cranial nerve compression at root entry or exit zone (REZ) giving rise to varied symptoms. It can also result secondary to compression by a tumor or a demyelinating disease such as multiple sclerosis.1 The most commonly affected nerve is trigeminal nerve, followed by facial nerve, glossopharyngeal nerve, and vestibulocochlear nerve, resulting in trigeminal neuralgia (TN), hemifacial spasms (HFSs), glossopharyngeal neuralgia, and vestibular paroxysmia.2
Prevalence of TN is 0.1 to 0.2 per 1,000 patients, while the incidence ranges from 4 to 5 per 100,000 per year to 20 per 100,000 per year in patients above 60 years.3 It is characterized by spontaneous unilateral, lancinating pain evoked by external stimuli lasting for a few seconds or minutes. Maxillary (V2) and mandibular (V3) divisions of TN are commonly affected. Although medical management is the preferred choice, in case of refractory TN, a neurosurgical procedure including microvascular decompression (MVD), percutaneous procedures, or radiosurgical procedure is recommended. MVD is associated with early and long-term pain relief in 68 to >90% of the cases.4
Prevalence of HFS is 11 per 100,000 population with an incidence of 0.77 per 100,000 per year.2 It is characterized by unilateral, progressive, involuntary movements of at least one muscle of facial expression. The symptoms persist even during sleep and are associated with psychological stress and decreased quality of life. Over the years, long-standing HFS may result in low-grade facial nerve paralysis. Management of HFS ranges from symptomatic treatment including heat application, medical management, and botulinum injections to treatment of cause such as microvascular surgery.5 Success rate of MVD in hemifacial surgery is 80 to 88%.6
NVSs pose a challenge to neurosurgeons as it requires accurate diagnosis and timely management. Most patients would have missed diagnosis and taken inappropriate treatment. Because of its varied clinical profile in different age groups, a thorough knowledge of their pathology and natural history is must for all neurologists and neurosurgeons. In view of this, we conducted this study with an objective to describe and correlate the clinical, radiological, and intraoperative findings in patients with refractory TN and HFSs not responding to conventional medical management. The secondary objective was to determine the outcome and complications related to MVD in these patients.
Materials and Methods
In this retrospective study, the clinical profile, radiographic features, intraoperative findings, and surgical outcome of 17 patients with NVS who underwent surgery for symptom relief from January 2018 to July 2021 were assessed. Patients with refractory TNs and HFSs with severely debilitating symptoms obstructing daily activities and who did not respond to initial medical management or other interventions were selected for surgical procedure. The procedure was explained in detail to the patient, and informed consent was obtained prior to the surgical procedure.
Operative Procedure
Patients were laterally positioned with head firmly secured on four-headpin frame. Retromastoid suboccipital craniotomy was done in all patients with superior exposure to transverse and sigmoid sinus. After gently retracting the cerebellum, the cerebrospinal fluid (CSF) was gently released from the cerebello-medullary cistern. As an entangled arachnoid keeps these vessels in proximity to the cranial nerve, careful releasing of the arachnoid was done to free the vessels transmitting their pulsation onto the cranial nerve. Similarly, MVD through the retromastoid suboccipital craniotomy approach was done in patients with vascular compression. The offending vessel was kept aside using shredded Teflon felt around the trigeminal nerve. However, the patients with nerve compression secondary to tumor underwent tumor decompression through the retro mastoid approach. One patient in whom multicompartment epidermoid was present underwent frontotemporal craniotomy, transsylvian approach, and tumor decompression. Following surgery, patients were followed up for 3 to 36 months, and relief of symptoms was assessed.
Data Collection and Statistical Analysis
Variables including demographics (age, sex), clinical features (site, duration of symptoms, distribution), magnetic resonance imaging (MRI) findings, micro-neurosurgical details (type of surgery, obstructive vessel), and postoperative outcome and complications were recorded. The identified data were entered and stored on excel spreadsheets and imported to Statistical Package for Social Sciences software (SPSS version 20, Chicago, Illinois, United States) for analysis. Descriptive analysis was performed. Continuous variables were presented as mean and standard deviation, while categorical variables were presented as frequency percentages.
Results
Demographic and Clinical Findings
In our series, 14 patients had refractory TN, and three patients had HFSs. Characteristics of patients with TN and HFS are summarized in Table 1. The study cohort consisted of 17 patients (9 females and 8 males). While all patients with HFS were males, TN was common in females (64.3%). Patients belonged to the age range of 28 to 72 years with a median age of 56 years. The mean (standard deviation) age of patients in our study cohort was 52.6 (12.2) years. The duration of symptoms varied from 8 months to 11 years, with a mean of 2.8 (0.9) years. The mean duration of symptoms was longer in patients with HFSs than in patients with TN (3.3 vs. 2.7 years). While the right side was commonly affected in TN (64.3%), the left side was common in HFS (66.7%). The predominant complaint was severe facial pain along the trigeminal nerve distribution, not responding to conventional medicines or partial response to carbamazepine. The most common neuralgia symptoms were distributed along the v2v3 (maxillary and mandibular division) branches (42.9%). One patient, along with neuralgia, had mild ataxia and unilateral weakness of limbs. Two patients had recurrent HFSs causing significant disability while speaking. Three patients had a severe sensorineural hearing loss on the side of the pathology.
|
Variable |
Trigeminal neuralgia (n = 14) |
Hemifacial spasm (n = 3) |
All (n = 17) |
|---|---|---|---|
|
Age (in years) |
|||
|
Mean (SD) |
52.3 (12.3) |
55.7 (6.03) |
52.6 (12.2) |
|
Median (min–max) |
6.5 (28–72) |
55 (50–62) |
56 (28–72) |
|
Gender, n (%) |
|||
|
Male |
5 (35.7) |
3 (100) |
8 (47.1) |
|
Female |
9 (64.3) |
0 |
9 (52.9) |
|
Side, n (%) |
|||
|
Right |
9 (64.3) |
1 (33.3) |
10 (58.8) |
|
Left |
5 (35.7) |
2 (66.7) |
7 (41.2) |
|
Duration (in years) |
|||
|
Mean (SD) |
2.7 (2.7) |
3.3 (3.2) |
2.8 (2.9) |
|
Median (min–max) |
1.5 (0.08–11) |
2 (1–7) |
2 (0.08–11) |
Abbreviations: max, maximum; min, minimum; n, number; %, percentage; SD, standard deviation.
Radiographic Findings
Out of 17 patients, 9 patients showed definite neurovascular conflict. In most patients, the superior cerebellar artery (SCA) was found to be closely abutting the root exit zone of the trigeminal nerve unilaterally. In two patients who had severe HFSs, there was an aberrant high lying tortuous vertebral artery close to the facial nerve, which was clearly appreciated on MRI (Fig. 1A). MRI images of three patients showed epidermoid tumor along the cerebellopontine angle, compressing the trigeminal nerve (Fig. 1B). Among them, one patient had extensive epidermoid tumor involving the middle cranial fossa and posterior fossa compressing the brainstem. In two patients, MRI revealed classical vestibular schwannoma with the epicenter on internal acoustic meatus. In three patients with TN, there was crowding of posterior fossa and short cisternal segment of the trigeminal nerve. Also, there was Arnold Chiari malformation with syringomyelia in one case. (Fig. 1C, D and Table 2).
|
Variable |
Frequency, n (%) |
|---|---|
|
MRI findings |
|
|
Vessel conflict |
9 (52.9) |
|
Short cisternal segment |
3 (17.6) |
|
Cerebellopontine angle epidermoid tumor |
3 (17.6) |
|
Cerebellopontine vestibular schwannoma |
2 (11.8) |
|
Surgery |
|
|
MVD |
12 (70.6) |
|
Craniotomy and decompression |
5 (29.4) |
|
Intraoperative findings |
|
|
Compression of SCA |
4 (23.5) |
|
Compression of SCA + premeatal segment of AICA |
2 (11.8) |
|
Short cisternal segment, crowded posterior fossa |
3 (17.6) |
|
Vertebral artery |
2 (11.8) |
|
AICA near facial nerve |
1 (5.9) |
|
Lesion causing compression |
5 (29.4) |
|
Complications |
|
|
None |
13 (76.5) |
|
Transient facial nerve palsy |
1 (5.9) |
|
Diplopia secondary to trochlear nerve paresis |
1 (5.9) |
|
Death secondary to chest infection |
1 (5.9) |
|
Paradoxical CSF rhinorrhea and facial paresis mild |
1 (5.9) |
|
Follow-up in months |
|
|
Mean (SD) |
12.7 (11.8) |
|
Median (min–max) |
12 (1–36) |
|
Relief of symptoms |
|
|
Immediate |
15 (88.2) |
|
Delayed |
2 (11.8) |
Abbreviations: AICA, anterior inferior cerebellar artery; CSF, cerebrospinal fluid; max, maximum; min, minimum; MRI, magnetic resonance imaging; MVD, microvascular decompression; n, number; %, percentage; SCA, superior cerebellar artery; SD, standard deviation.
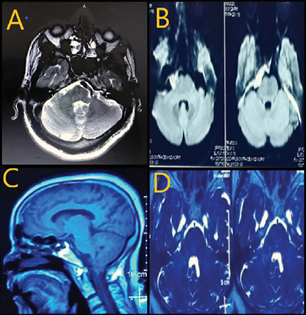
-
Fig. 1 MRI images depict various causes for neurovascular syndrome. (A) Ectatic vertebral artery in close proximity to facial nerve. (B) Epidermoid tumor in crural cistern. (C) Tonsillar herniation in Chiari presenting with trigeminal neuralgia. (D) Short cisternal segment of trigeminal nerve. MRI, magnetic resonance imaging.
Fig. 1 MRI images depict various causes for neurovascular syndrome. (A) Ectatic vertebral artery in close proximity to facial nerve. (B) Epidermoid tumor in crural cistern. (C) Tonsillar herniation in Chiari presenting with trigeminal neuralgia. (D) Short cisternal segment of trigeminal nerve. MRI, magnetic resonance imaging.
Intraoperative Findings
Among the 17 patients, 12 patients underwent MVD through the retromastoid suboccipital craniotomy approach (Table 2). In all patients of TN, the SCA was the major offending vessel causing irritation or compression of trigeminal nerve near REZ. Additionally, the premeatal segment of the AICA was also seen in close relation with the trigeminal nerve in two patients (Fig. 2 and Table 2). In patients with TN secondary to lesions, along with the fifth nerve compression, twisted and entangled arachnoid was noted, which contributed to the vessel compression onto the cranial nerves causing severe neuralgia and facial spasms. In HFS patients, a tortuous vertebral artery was seen in close proximity to the facial nerve in two patients and AICA in one patient (Fig. 3A, B). Four patients underwent tumor decompression through the retromastoid approach for epidermoid tumors, while one patient underwent frontotemporal craniotomy, transsylvian approach, and tumor decompression for management of multicompartment epidermoid tumor (Fig. 3C, D).
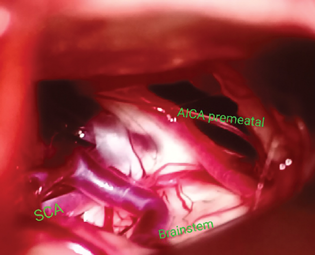
-
Fig. 2 Intraoperative picture of trigeminal neuralgia showing nerve compression ventrally by superior cerebellar artery, dorsally by anterior inferior cerebellar artery, and venous loops near root entry zone of trigeminal nerve.
Fig. 2 Intraoperative picture of trigeminal neuralgia showing nerve compression ventrally by superior cerebellar artery, dorsally by anterior inferior cerebellar artery, and venous loops near root entry zone of trigeminal nerve.
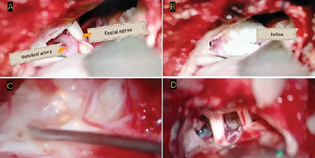
-
Fig. 3
(A) Intraoperative picture of hemifacial spasm showing a large dolichoectatic vertebral artery abutting the facial nerve. (B) Teflon being insulated between facial nerve and vertebral artery. (C) Intraoperative picture of cerebellopontine angle epidermoid completely engulfing the neurovascular bundles near brain stem. (D) Post decompression of epidermoid 5th and 7/8th cranial nerves are seen.
Fig. 3 (A) Intraoperative picture of hemifacial spasm showing a large dolichoectatic vertebral artery abutting the facial nerve. (B) Teflon being insulated between facial nerve and vertebral artery. (C) Intraoperative picture of cerebellopontine angle epidermoid completely engulfing the neurovascular bundles near brain stem. (D) Post decompression of epidermoid 5th and 7/8th cranial nerves are seen.
The MVD was challenging in three patients. In the first patient with a previous history of MVD and gamma knife radiosurgery done 5 years ago, the presence of thick arachnoid and more adhesions was challenging. Moreover, there were thick multiple tributaries of superior petrosal vein engulfed in thick arachnoid, making surgical corridor toward trigeminal nerve very difficult. In a patient with Arnold Chiari malformation and syringomyelia, large retromastoid craniotomy was done till the base to include foramen magnum. Cerebellum was full, and enough CSF could not be released due to the crowded posterior fossa. After gentle traction on the cerebellum, a very short cisternal segment of the trigeminal nerve was appreciated. Thickened arachnoid around the trigeminal nerve was released all around, which made the nerve relax. In another patient, the origin of the trigeminal nerve was just above the tentorium and was associated with a thickened arachnoid at its REZ.
Outcome and Follow-Up
In our study, the patients were followed up for a period of up to 36 months, with a mean follow-up of 12.7 months. Almost all patients (88.2%) reported immediate postoperative complete pain relief. Two patients had delayed relief of symptoms and were prescribed carbamazepine and baclofen. While one patient with vestibular schwannoma developed paradoxical CSF rhinorrhea, which subsided after re-exploration and sealing off the mastoid air cells with bone wax and fibrin glue, another patient developed transient facial nerve paresis following surgery. Following surgery, a mild trochlear nerve paresis was reported in another patient with TN, where the fifth nerve was arising above the tentorium. The patient recovered within a week. One patient in whom multicompartment epidermoid was operated died 4 weeks after surgery as she developed a chest infection. She had lower cranial nerve involvement with severe brain stem compression (Table 2).
Discussion
By definition, NVS results either from direct compression or irritation of cranial nerves by the vascular loop near REZ.7 The Obersteiner–Redlich zone (REZ) is the transition zone between central and peripheral myelin, wherein the oligodendroglial cells are transitioned to Schwann cells to form peripheral myelin, and is susceptible to mechanical vulnerability.8 Interestingly, the length and location of REZ vary between the cranial nerves. Occasionally these areas get in close contact with an aberrant course of a vessel, predominantly artery.2 9 Due to the tortuous course, higher pressure and pulsatility, these close proximities of arteries can irritate the cranial nerve resulting in various symptoms. Although rare, the presence of space-occupying intracranial lesions at the cerebellopontine angle and those close to the cranial nerve, including meningiomas, epidermoid tumor, schwannomas, and arachnoid cysts, also causes compression resulting in symptomatic neuropathy.10 Various cranial neuropathies secondary to vascular compression are defined. They include TN (trigeminal nerve), HFS (facial nerve), disabling positional vertigo, also called vestibular paroxysmia (vestibulocochlear nerve), and torticollis of the accessory nerve origin. Among these, the incidence of TN and HFS is higher than others.
Although TN is a well-known condition, nonetheless is often misdiagnosed due to overlapping symptoms in the orofacial region. According to Tripathi et al,11 more than 40% of patients with TN undergo dental extraction due to misdiagnosis. The rarity of the reported and treated cases is attributed to the missed diagnosis and inappropriate treatment before referral to neurologists and neurosurgeons. Hence, most patients with TN suffer severe pain before arriving at an accurate diagnosis. The underlying pathophysiology is related to ephaptic transmission in the trigeminal nerve from partially demyelinated large A-fibers to thinly myelinated A-delta and C (nociceptive) fibers.12 Although carbamazepine is effective as initial therapy with a good response to alleviate the severe lancinating paroxysmal electric shock-like pain,13 14 there is a tendency for spontaneous remissions with pain-free intervals for weeks or months. Around 75% ultimately fail to medical therapy. Moreover, due to the severity of pain, suicidal tendencies are common among these patients during the course of the illness.15 In such patients, surgical intervention, specifically MVD, is advised to treat the root cause of the disease.16
Another rare condition affecting the muscles of facial expression is HFS, which is characterized by recurrent involuntary painless contractions.17 The main causative factor of HFS is the compression of the facial nerve by a vessel in the brainstem near the REZ. Although conservative management and botulinum toxin injection are effective initially to treat HFS, MVD is the treatment of choice to prevent facial nerve paralysis and provides long-term relief of symptoms and lowest recurrence.4 In our series, 14 patients of TN and three patients of HFS refractory to medical management were included. In TN patients, nine patients had pain along the right half of the face (64.3%), mainly involving V2V3 distribution (42.9%). No sex predilection was seen. This observation is in accordance with earlier literature.18 Incidence of HFS is common in women at the ages between 30 to 50 years, and has an affinity for left side.5 In our study cohort, the left side was commonly affected; however, all three patients were males. This difference could be due to the smaller sample and inclusion criteria set for surgical procedures.
TN is broadly classified into two categories. One is idiopathic or classical TN, and the other is secondary TN. According to the literature, most idiopathic or classical TN cases are due to a neurovascular conflict at the REZ.19 Secondary ones are due to space-occupying lesions like posterior fossa epidermoid, vestibular schwannoma, meningioma, and multiple sclerosis. Occasionally demyelinating plaques in multiple sclerosis can cause refractory TN with poorer outcomes to surgical techniques.20 Similarly, primary HFS is caused by vascular decompression, and secondary HFS is due to facial nerve paralysis.21 In our study, HFS in all patients and TN in 12 patients was due to vascular compression, while TN in five patients was secondary to an epidermoid tumor and vestibular schwannoma at the cerebellopontine angle.
SCA is the most common offending vessel (80%) in TN, followed by AICA, dolichoectatic basilar artery and venous loops.22 In HFS, AICA, rarely by elongated SCA, PICA, tortuous vertebral artery, and dolichoectatic basilar artery compress the facial nerve.23 In this study, SCA was the main offending vessel in TN followed by AICA and venous compression. Interestingly, in two patients of HFS, we also noted a tortuous vertebral artery running in close proximity to the facial nerve, and in another patient, AICA was seen along the thickened entangled arachnoid. Previous studies have suggested good clinical outcomes in patients with TN (68 to >90%) and HFS (80–88%) following MVD surgery.4 6 In our study, 88.2% of patients had immediate relief. One patient with an epidermoid tumor succumbed to chest infection 4 weeks postsurgery.
Irrespective of the causal classification of TN and HFS into idiopathic and secondary, the pathophysiology remains the same. Results of this study suggest that alteration of neurovascular bundles can be present in space-occupying lesions as well. During decompression of the lesions, we observed a neurovascular conflict even in these patients, which could be due to the mechanical compression in the posterior fossa by the tumor, which would have stretched and brought the vessels more in contact with the cranial nerve. Moreover, the literature also suggests a lack of definite evidence of obvious vascular compression in some patients of TN and HFS.24 Therefore, significant relief postsurgery in such patients cannot be attributed only to vascular compression alone.
The symptoms in these patients can be correlated to the arachnoid entanglement theory, which was a constant finding in patients with or without vascular compression. Arachnoid entanglement holds on to the vessel and the cranial nerve giving rise to constant transmission of the pulsation. This theory explains the pulsation transmission of other vessels that are not in direct contact but do transmit the pulsation in the posterior fossa.25 This phenomenon was confirmed in a case of severe TN who also had Arnold Chiari malformation with syringomyelia in this study cohort. In this case, we observed extreme crowding of the posterior fossa contents and thickened arachnoid was tight all around and with minimal CSF in the cisterns. Moreover, the cisternal segment of the trigeminal nerve was also very short. Releasing the arachnoid in and around the fifth nerve and decompression of posterior fossa in terms of craniotomy and dural opening would have eased the tension over the cranial nerves and vessels under subarachnoid space. In another case of refractory TN, the origin of the fifth nerve was just above the tentorium cerebelli. Notably, MRI findings suggested a short cisternal segment of the nerve and associated crowding of the posterior fossa contents. During surgery, we observed that REZ of the fifth nerve was above the tent engulfed in thick arachnoid, and we observed the trochlear nerve before appreciating the origin of the trigeminal nerve. Since the entangled arachnoid keeps the vessels in proximity to the cranial nerve, careful releasing of the arachnoid freed most of the vessels, transmitting their pulsation onto the cranial nerve.
Analyzing these complex cases, we feel it is not the compressing vessel alone but overall crowding at the posterior fossa with arachnoid entanglement which determines the root cause of severe neuralgias. Despite these positive findings, our study is not without limitations, including the study's retrospective nature and its associated inherent limitations—smaller sample size with nonuniform distribution and limited interferential statistical analysis. Comparison of pain scores pre- and postsurgery could have added patients' perspectives on the surgical procedure. Therefore, further prospective studies with a higher sample size are warranted.
Conclusion
NVSs are quite debilitating conditions that need comprehensive evaluation of clinical profile and imaging. Despite having typical characteristic features, TN is often misdiagnosed or diagnosed much later in clinical practice. Advanced micro-neurosurgical techniques used in MVD achieve excellent outcomes with improved quality of life. During MVD, the neurosurgeon should focus not only on releasing the vessel away from the cranial nerve, but also on the thorough release of arachnoid all around the REZ. As arachnoid entanglement and vascular compression over the cranial nerves are the leading causes of NVS, the option of surgery should be offered in the early part of their clinical course of all NVSs.
Conflict of Interest
None declared.
References
- Chronic facial pain: trigeminal neuralgia, persistent idiopathic facial pain, and myofascial pain syndrome-an evidence-based narrative review and etiological hypothesis. Int J Environ Res Public Health. 2020;17(19):7012.
- [Google Scholar]
- Imaging of neurovascular compression syndromes: trigeminal neuralgia, hemifacial spasm, vestibular paroxysmia, and glossopharyngeal neuralgia. AJNR Am J Neuroradiol. 2016;37(8):1384-1392.
- [Google Scholar]
- Epidemiology of typical and atypical craniofacial neuralgias. Neurol Sci. 2005;26:s65-s67. (Suppl 2):
- [Google Scholar]
- Nerve compression syndromes in the posterior cranial fossa. Dtsch Arztebl Int. 2019;116(4):54-60.
- [Google Scholar]
- Hemifacial spasm and neurovascular compression syndrome. BMJ Case Rep. 2017;2017:bcr2016218883.
- [Google Scholar]
- Hemifacial spasm: conservative and surgical treatment options. Dtsch Arztebl Int. 2012;109(41):667-673.
- [Google Scholar]
- The neurovascular syndromes: a review of pathophysiology - lessons learnt from Prof. Chandy's paper published in 1989. Neurol India. 2019;67(2):377-388.
- [Google Scholar]
- Trigeminal neuralgia caused by venous angioma: a case report and review of the literature. World Neurosurg. 2015;84(3):860-864.
- [Google Scholar]
- Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery. 2006;59(2):354-359. , discussion 354–359
- [Google Scholar]
- The many faces of hemifacial spasm: differential diagnosis of unilateral facial spasms. Mov Disord. 2011;26(9):1582-1592.
- [Google Scholar]
- Please spare my teeth! Dental procedures and trigeminal neuralgia. Surg Neurol Int. 2020;11:455.
- [Google Scholar]
- Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology. 1984;34(4):418-426.
- [Google Scholar]
- The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629-808.
- [Google Scholar]
- Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol Pain. 2020;16:1744806920901890.
- [Google Scholar]
- Harvey Cushing's case series of trigeminal neuralgia at the Johns Hopkins Hospital: a surgeon's quest to advance the treatment of the ‘suicide disease’. Acta Neurochir (Wien). 2011;153(5):1043-1050.
- [Google Scholar]
- Microvascular decompression as a surgical management for trigeminal neuralgia: a critical review of the literature. Neurol India. 2009;57(2):134-138.
- [Google Scholar]
- Trigeminal neuralgia: frequency of occurrence in different nerve branches. Anesth Pain Med. 2011;1(2):70-72.
- [Google Scholar]
- Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology. 2016;87(2):220-228.
- [Google Scholar]
- Trigeminal neuralgia secondary to multiple sclerosis: from the clinical picture to the treatment options. J Headache Pain. 2019;20(1):20.
- [Google Scholar]
- Hemifacial spasm and neurovascular compression. ScientificWorldJournal. 2014;2014:349319.
- [Google Scholar]
- Microvascular decompression on patients with trigeminal neuralgia caused by ectatic vertebrobasilar artery complex: technique notes. Acta Neurochir (Wien). 2012;154(5):793-797. , discussion 797
- [Google Scholar]
- Correlation between idiopathic hemifacial spasm and the MRI characteristics of the vertebral artery. J Clin Neurosci. 2011;18(4):528-530.
- [Google Scholar]
- Trigeminal neuralgia without neurovascular compression presents earlier than trigeminal neuralgia with neurovascular compression. J Neurosurg. 2015;123(6):1519-1527.
- [Google Scholar]
- Arachnoid membranes around the cisternal segment of the trigeminal nerve: a cadaveric anatomic study and intraoperative observations during minimally invasive microvascular decompression surgery. World Neurosurg. 2019;125:e262-e272.
- [Google Scholar]







