Translate this page into:
Cognitive and Functional Outcomes following Inpatient Rehabilitation in Patients with Acquired Brain Injury: A Prospective Follow-up Study
Address for correspondence: Dr. Anupam Gupta, Department of Neurological Rehabilitation, National Institute of Mental Health and Neurosciences, Bengaluru - 560 029, Karnataka, India. E-mail: drgupta159@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives:
To study the effects of cognitive retraining and inpatient rehabilitation to study the effects of cognitive retraining and inpatient rehabilitation in patients with acquired brain injury (ABI).
Design and Setting:
This was a prospective follow-up study in a neurological rehabilitation department of quaternary research hospital.
Patients and Methods:
Thirty patients with ABI, mean age 36.43 years (standard deviation [SD] 12.6, range 18–60), mean duration of illness 77.87 days (SD 91.78, range 21–300 days) with cognitive, physical, and motor-sensory deficits underwent inpatient rehabilitation for minimum of 14 sessions over a period of 3 weeks. Nineteen patients (63%) reported in the follow-up of minimum 3 months after discharge. Type of ABI, cognitive status (using Montreal Cognitive assessment scale [MoCA] and cognitive Functional Independence Measure [Cog FIM]®), and functional status (motor FIM®) were noted at admission, discharge, and follow-up and scores were compared.
Results:
Patients received inpatient rehabilitation addressing cognitive and functional impairments. Baseline MoCA, motor FIM, and Cog FIM scores were 15.27 (SD = 7.2, range 3–30), 31.57 (SD = 15.6, range 12–63), and 23.47 (SD = 9.7, range 5–35), respectively. All the parameters improved significantly at the time of discharge (MoCA = 19.6 ± 7.4 range 3–30, motor FIM® = 61.33 ± 18.7 range 12–89, Cog FIM® =27.23 ± 8.10 range 9–35). Patients were discharged with home-based programs. Nineteen patients reported in follow-up and observed to have maintained cognition on MoCA (18.8 ± 6.8 range 6–27), significantly improved (P < 0.01) on Cog FIM® (28.0 ± 7.7 range 14–35) and motor FIM® =72.89 ± 16.2 range 40–96) as compare to discharge scores.
Conclusions:
Cognitive and functional outcomes improve significantly with dedicated and specialized inpatient rehabilitation in ABI patients, which is sustainable over a period.
Keywords
Acquired brain injury
cognitive retraining
follow-up
functional recovery
inpatient rehabilitation
INTRODUCTION
Acquired brain injury (ABI) is defined as “damage to the brain, which occurs after birth and is not related to a congenital or a degenerative disease.” “These impairments may be temporary or permanent and cause physical, functional disability, or psychosocial maladjustment.”[12] By this definition, ABI encompasses a wide variety of disorders of varying etiologies such as vascular, hypoxic, malignant, and traumatic. There are often long-lasting effects on domains of cognition, motor, behavior, and personality in affected individuals.[3] Cognitive impairment is common sequelae and important marker for prediction of rehabilitation outcomes, and cognitive outcome can be modified through targeted interventions.[4]
Studies suggest that traumatic brain injury (TBI) and stroke are the two main causes of ABI and regarded as important public health problem.[5] The incidence of TBI from 23 reports was found to vary greatly among European countries. Most rates were in the range 150–300/100,000 people per year.[6] The prevalence of stroke In western developed world ranges from 500 to 600/100,000. Rates per 100,000 from developing countries are also variable and range from 58 in India and 76 in the United Republic of Tanzania to 620 in China and 690 in Thailand.[7] Between 1.5 and 2 million persons are injured and 1 million die every year in India following TBI.[8] Cardiovascular diseases including stroke caused 19% of deaths in India between 2001 and 2003 and this is estimated to rise to 36% by 2030.[9] According to disease burden in India report September 2005, central nervous system malignancies (included in ABI) comprise 2% of the total cancer burden.[10] Other causes of ABI such as meningoencephalitis and stroke mimics also contribute to this pool of patients.
The majority of ABI survivors continue to live with disabilities without access to comprehensive rehabilitation services and remain a burden on caregivers and society.[1112] Physical and cognitive deficits are most commonly observed in these patients but are not adequately addressed due to lack of approachable rehabilitation services and awareness.[1314] Many of these patients opt for complementary and alternative medicine, which are popular in India but demonstrate questionable benefits.[15]
It is evident, both clinically and scientifically, that the improvement in motor control after ABI is training dependent, responding best to repetitive task training with continuous modification of the program to keep training tasks challenging to the patients (activity-based recovery and neural plasticity).[1617] Single or multiple domains of cognition can be affected in these patients depending on the site (s) and severity of injury. Disturbances in memory, attention, and/or executive functions are commonly involved. Deficits in language and speech, learning, abstract thinking, and orientation occur in severe cases. It is well established that cognitive deficits interfere with rehabilitation efforts and also result in a greater negative impact on quality of life.[18] Cognitive rehabilitation (CR) is a specialized treatment procedure designed to improve the cognition affected by internal or external injury to the brain. There are two types of CR: Restorative and compensatory rehabilitation.[192021] Restorative rehabilitation enables the patient to develop lost functions through specialized computerized and manual cognitive exercises. Compensatory rehabilitation helps the patient to train and use aids and tools to overcome the impairment. The objective of the present study was to rehabilitate ABI patients in all affected domains including cognitive, physical, sensory-motor, and behavior with customized inpatient programs. Another objective was to observe the effect of inpatient rehabilitation in improving cognition and functionality of the patients (by comparing admission and discharge scores). We also tried to observe whether the benefits of inpatient rehabilitation are sustainable by assessing the patients in follow-up examination a minimum of 3 months after discharge.
PATIENTS AND METHODS
The study was conducted in neurorehabilitation department of a quaternary university research hospital between September 2015 and April 2016. Adult patients aged between 18 and 65 years with ABI who reported to the department for inpatient Rehabilitation minimum 3 weeks post- injury were included. Informed consent was taken from all the patients, or their caregivers, before including them in the study. The study was approved by the Institute's Ethics Committee. Patients with global aphasia, poor comprehension, or comorbidities interfering with rehabilitation were excluded from the study. Some patients who could not participate after few sessions of starting interventions, either due to deterioration or other medical comorbidities, were also excluded from final analysis.
A total of 48 patients with ABI were admitted for rehabilitation in the department during the study period. Out of these, 30 patients who met the inclusion criteria and who completed the sessions were included for final analysis. Nine patients were excluded because of severe cognitive deficits affecting their participation, 4 were excluded because of reduced comprehension for a simple verbal task, 2 patients excluded due to deterioration of their condition, and 3 patients did not complete all 14 sessions.
All patients were evaluated with detailed clinical examination and sociodemographic data were recorded. The following scales were used to record cognitive and functional status. Cognitive assessment was done using Montreal Cognitive Assessment Scale (MoCA), and the Cognitive Functional Independence Measure (Cog FIM®). Functional status was assessed using FIM (Motor FIM®) for self-care, sphincter, mobility, communication, and psychosocial aspects.
The rehabilitation program started after completing the initial clinical assessment within 24 h of admission. All the patients underwent comprehensive rehabilitation for a minimum of 14 sessions over a period of 3 weeks with multidisciplinary training. Physical and occupational therapy sessions were conducted for 1 h each, 6 days a week, and were usually customized according to the patients’ need.
Physiotherapy is focused on activity-based training to improve locomotion, spasticity reduction, improvement in balance, and lower-limb coordination depending on requirements. Physiotherapy also consisted of positioning, resistive and stretching exercises, and graded gait training.
Occupational therapy is focused on improving the functional abilities including fine motor hand skills training, coordination activities with upper extremity, and activities of daily living (ADL). These therapies consisted of range of motion, positioning, facilitation techniques, and ADL retraining with compensatory techniques. Appropriate splints were provided to the patients wherever required.
Initial cognitive assessment was done by the clinicians. Later, detailed cognitive assessment and retraining were conducted by psychologists who were part of the rehabilitation team. “National Institute of Mental Health and Neuro Sciences Neuropsychological test battery” was used to assess basic cognitive functions such as motor and mental speed, attention (focused, sustained, and divided), comprehension, visuospatial construction, learning, and memory as well as executive functions such as fluency (verbal, category, and design), working memory, planning set shifting, and response inhibition.[22] After detailed assessment was completed within 48 h of admission, cognitive retraining was imparted focusing on the areas of cognitive impairment. A total of 14 number sessions were divided into cognitive assessment and cognitive retraining. The initial 2 sessions were devoted for assessment and 12 for cognitive retraining. The duration for cognitive assessment varied with each session going on for up to 3–4 h and the duration of each retraining session also varied depending on the patient's participation. Retraining sessions which could not be conducted due to the patients’ condition/discomfort or lasting <30 min were not considered as “sessions.” Retraining consisted of traditional methods as well as a computer-based retraining program tailored according to patient's clinical status and need. Patients were evaluated with the same scales at the time of discharge for cognition and functional status after a minimum of 14 sessions and the scores were compared.
At the time of discharge, patients were advised home-based program and asked to report for follow-up in neurorehabilitation outpatient services after 3 months. Nineteen patients (63%) reported in the follow-up after 3 months. These patients were reevaluated for cognitive and functional status and the respective scores were noted and compared with the discharge scores.
Outcome measures
Functional independence measure®
FIM is a widely used index of rehabilitation outcome that measures the level of assistance that an individual requires to perform basic life activities.[23] It is an 18-item, 7-level scale that rates the ability of a person to perform independently in self-care, sphincter control, transfers, locomotion, communication, and social activity. Total score is obtained by summing the scores range from 18 (maximally dependent) to 126 (maximally independent). Two motor and cognitive subscales can be obtained by summing the 13 motor items (range, 13–91) and the 5 cognitive items (range, 5–35).
The Cog FIM subscale is a part of the global FIM assessment and comprised 2 items (communication and social cognition) that relate to cognitive functions such as comprehension, expression, social interaction, problem-solving, and memory. A score of 35 points represents optimal performance. A significant positive correlation has been found with FIM and Mini–Mental State Examination (MMSE).[24] The FIM scale was purchased for this particular study.
Montreal cognitive assessment scale
MoCA is a screening tool for cognition and is found to be more sensitive and specific for the detection of mild cognitive deficits compared to Folstein's MMSE.[25] MoCA tests visuospatial executive, naming, memory, attention, language, abstraction, delayed recall, and orientation aspects of cognition. The total MoCA score is obtained by summing the scores of all domains from 0 to 30. Scores <10 indicate severe, 10–17 moderate, and scores between 18 and 26 denote mild cognitive impairment. A score >26 is interpreted as normal cognition.
Data analysis
Data were analyzed using the Statistical Package for the social science statistical package SPSS version 22.0 (IBM, IL, Chicago, USA).
Initially, repeated ANOVA measures were applied and found to have a statistically significant effect of time on cognitive and functional status after the retraining program at three-time points; admission, discharge, and follow-up. Paired t-tests were applied to compare pre- versus post-retraining scores and postretraining scores compared with follow-up scores.
RESULTS
Diagnosis, sociodemographics, and comorbidities
Out of the 30 patients included in the study, 19 had stroke with right or left hemiplegia, 8 had TBI, and 3 patients had brain tumors. A total of 19 patients reported after 3-month follow-up (10 with stroke, 7 with TBI, and 2 with tumor).
Mean age of the patients was 36.3 years (standard deviation [SD] 12.8, range 18–60). Twenty-four were males (80%) and six were females. Twelve patients had hypertension and 4 had diabetes. The mean duration of illness was 72.1 days (SD 95.1, range 25–300) and mean length of stay in the rehabilitation unit was 31.3 days (SD 9.1, range 20–51).
Changes after inpatient rehabilitation and follow-up scores
MoCA, motor FIM, and Cog FIM results improved significantly at discharge after inpatient rehabilitation in the form of functional and cognitive retraining when compared with admission. During the follow-up after 3 months, cognition was more or less same, but there was further significant motor recovery observed in the patients. Data are shown in Table 1.

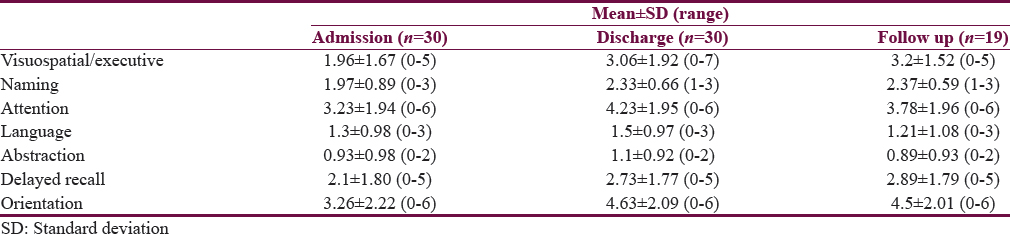
Table 2 describes cognitive domain-wise mean ± SD scores. All domains show improvement. Orientation was improved the most and abstraction the least.
DISCUSSION
The present study was conducted to evaluate the benefits of multidisciplinary inpatient rehabilitation in ABI patients on cognition and functionality. Cognitive and functional impairment improved in our study after inpatient rehabilitation with focused, customized cognitive, and functional retraining. The effects were maintained after 3 months of discharge when the patients were followed up in outpatient department (OPD). This is in line with several recent studies suggesting that multidisciplinary inpatient rehabilitation is beneficial for ABI patients, with improvement in the level of self-care and mobility, cognition, and time to return to work. This has been found not only in subacute but also in the chronic phase.[18262728]
Effect on cognition
After ABI, cognition can be affected in single or multiple domains. Tatemichi et al. identified cognitive problems in attention, memory, language, and orientation along with deficits in visuospatial skills and abstract reasoning in a community-based comparison of stroke patients with population controls.[29] Marked deficits in abstraction, executive function, and processing speed were reported by Sachdev et al. in stroke patients.[30] Hence, there is no consistent profile of cognitive deficits in stroke though slowed information processing and executive dysfunction are predominant as suggested by Cumming et al.[31] Cognitive impairment is a common sequel of moderate and severe TBI affecting information processing speed, attention, memory, and executive functions.[3233] One can observe in the present study that, irrespective of cause of ABI, multiple domains of cognition are affected, which is easy to explain in cases with diffuse axonal injury. In cases of stroke with focal injury, the deficits depend on the site of injury, size of infarct/hemorrhage, and time of intervention.
The use of MoCA as a screening tool was chosen over MMSE for multiple reasons. MoCA has been validated as a screening tool for screening cognitive deficits in brain injury patients.[34] Pendlebury et al. studied the relationship between MoCA, Addenbrooke cognitive examination-revised (ACE-R), and MMSE and observed that MoCA and ACE-R have good sensitivity and specificity for mild cognitive impairment compared to MMSE.[35] ACE-R scale was not used in the study for several reasons; it is very elaborative, patients could not understand a lot of items mentioned in the scale, and we were not able to translate it in local vernacular language. The other advantage with MoCA is that it can be used in visually impaired patients as well as hemiparetic patients. While using MMSE as a screening tool, if a patient is right hand dominant and is having right side hemiparesis, domains such as copying and writing cannot be assessed. The patient's education is also not considered in MMSE, but while using MoCA, 1 point can be added to adjust for lesser years of education.[36] Hence, MoCA serves as an easy to apply, cost and time effective tool in busy OPDs for the screening of cognition. It is also an effective determinant for the requirement of detailed assessment and retraining.
In our study, all domains of MoCA were affected to some extent. At admission, 43.3% of patients had mild cognitive impairment, 33.3% moderate, 20% severe, and 3.3% had normal cognition. All domains of MoCA were affected variably at admission including visuospatial/executive, naming attention, and delayed recall. Abstract thinking was the most affected and showed minimal improvement when compared with other domains. At the time of discharge, 56% of patients had mild cognitive impairment, 20% moderate, 13.3% severe, and 10% had normal cognition on screening. When the mean scores of each domain of MoCA were compared, they showed improvement in all domains, with the maximum improvement being in orientation and least improvement in abstraction. This improvement in multiple domains’ postcognitive retraining is consistent with the results of a meta-analysis done by Cicerone et al. which showed substantial evidence in favor of interventions for attention, memory, social communication skills, and executive functioning with favorable results.[37] In our study, Cog FIM® scores also improved significantly with retraining.
At the time of follow-up, the patients maintained the gains in cognitive functions with no deterioration even after 3 months. Our study suggests that cognitive retraining is effective when administered in a strategic manner targeting deficit domains for retraining.
Effect on functional outcome
Lee et al. conducted a multi-time-point study to simultaneously compare changes in neurologic impairments (trunk control, motor function, sensory, and cognition) and recovery in functional impairments (activity of daily livings and gait) from the initiation of rehabilitation to 6 months after stroke. They found functional recovery was relatively rapid during the initial 4 weeks of treatment but decelerated between 3 and 6 months after stroke.[38] This study confirmed the importance of the period within 3 months of the stroke, during which most of the recovery occurs, emphasizing the need for rehabilitation at an early stage for recovery of impairments and functional performance. In our study, functional FIM® continued to improve even after discharge and significant improvement was observed at 3-month follow-up.
Many studies have shown the efficacy of inpatient rehabilitation programs on positive functional outcome in the subacute as well as the chronic phases in patients with ABI.[16383940] Tatemichi et al. demonstrated a substantial body of high-quality research evidence for the effectiveness and cost-effectiveness of rehabilitation in patients with ABI.[29] The improvement in functional status continues to occur if strategically planned inpatient rehabilitation is implemented in patients with severe TBI, even 3 months after injury.[41] Our study supports this evidence as reflected by significantly improved functional FIM scores after inpatient rehabilitation not only at the time of discharge but also at 3-month follow-up.
CONCLUSIONS
Patients with ABI can show a significant cognitive and functional recovery of deficits when treated with inpatient rehabilitation by an expert team in specialized neurorehabilitation centers. This recovery is observed across all of the domains of cognition and ADL skills irrespective of etiopathological diagnosis of brain injury. The recovery is significant but varies from patient to patient. This recovery can be sustained over a long period of time with efficient home-based programs. Our study also demonstrated that patients continue to improve functionally over time.
Limitations of the study
Patients were called in follow-up after 3 months of discharge. Eleven patients (37%) failed to report for unknown reasons. Although the cognitive retraining was customized as per the affected domains and patients, we were not able to analyze the effectiveness of a particular individual program in improving cognition. More studies with larger sample size and long-term follow-up are required to confirm that the improvements achieved with rehabilitation are maintained for longer term and help patients not only with ADLs but also in retaining premorbid status such as vocation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Brain Injury Association of America (BIAUSA). What is the Difference Between Acquired Brain Injury and Traumatic Brain Injury?. Available from: http://www.biausa.org/FAQRetrieve.aspx?ID=43913
- A systematic review of the rehabilitation of moderate to severe acquired brain injuries. Brain Injury. 2007;21:107-112.
- [Google Scholar]
- Rehabilitation Following Acquired Brain Injury: National Clinical Guidelines. London: Royal College of Physicians, British Society of Rehabilitation Medicine; 2003.
- Cognitive impairment in acquired brain injury: A predictor of rehabilitation outcomes and an opportunity for novel interventions. PM R. 2011;3(6 Suppl 1):S45-51.
- [Google Scholar]
- Challenges in understanding the epidemiology of acquired brain injury in India. Ann Indian Acad Neurol. 2015;18:66-70.
- [Google Scholar]
- A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien). 2006;148:255-68.
- [Google Scholar]
- Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43-53.
- [Google Scholar]
- Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24-8.
- [Google Scholar]
- National Commission on Macroeconomics and Health. Burden of Disease in India. New Delhi: Ministry of Health and Family Welfare, Government of India; 2005.
- Burden among stroke caregivers: Results of a community-based study from Kolkata, India. Stroke. 2010;41:2965-8.
- [Google Scholar]
- A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke. 1995;26:843-9.
- [Google Scholar]
- Incidence, types, risk factors, and outcome of stroke in a developing country: The Trivandrum Stroke Registry. Stroke. 2009;40:1212-8.
- [Google Scholar]
- Complementary and alternative medicine treatments among stroke patients in India. Top Stroke Rehabil. 2012;19:384-94.
- [Google Scholar]
- Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225-39.
- [Google Scholar]
- Rehabilitation interventions to improve locomotor outcome in chronic stroke survivors: A prospective, repeated-measure study. Neurol India. 2015;63:347-52.
- [Google Scholar]
- Neuropsychological Interventions: Clinical Research & Practice. New York: The Guilford Press; 2002.
- Sohlberg MM, Mateer CA, eds. Cognitive Rehabilitation: An Integrative Neuropsychological Approach (2nd edition). The Guilford Press; 2001.
- Psychological compensation: A theoretical framework. Psychol Bull. 1992;112:259-83.
- [Google Scholar]
- Practical and theoretical considerations in designing rehabilitation trials: The DVBIC cognitive-didactic versus functional-experiential treatment study experience. J Head Trauma Rehabil. 2006;21:179-93.
- [Google Scholar]
- NIMHANS Neuropsychology Battery-2004. Bangalore: NIMHANS Publication; 2004.
- Advances in functional assessment for medical rehabilitation. Top Geriatr Rehabil. 1986;1:59-74.
- [Google Scholar]
- Cognitive status at admission: Does it affect the rehabilitation outcome of elderly patients with hip fracture? Arch Phys Med Rehabil. 1999;80:432-6.
- [Google Scholar]
- Relationship between the montreal cognitive assessment and mini-mental state examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107.
- [Google Scholar]
- Motor recovery of stroke patients after rehabilitation: One-year follow-up study. Int J Neurosci. 2016;1:1-7.
- [Google Scholar]
- Functional improvement after 4-week rehabilitation therapy and effects of attention deficit in brain tumor patients: Comparison with subacute stroke patients. Ann Rehabil Med. 2015;39:560-9.
- [Google Scholar]
- Cochrane review: Multi-disciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database Syst Rev 2005:20. CD004170
- [Google Scholar]
- Cognitive impairment after stroke: Frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202-7.
- [Google Scholar]
- The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912-9.
- [Google Scholar]
- Stroke, cognitive deficits, and rehabilitation: Still an incomplete picture. Int J Stroke. 2013;8:38-45.
- [Google Scholar]
- Cognitive outcome following traumatic brain injury. J Head Trauma Rehabil. 2009;24:430-8.
- [Google Scholar]
- Changes in attention and information-processing speed following severe traumatic brain injury: A meta-analytic review. Neuropsychology. 2007;21:212-23.
- [Google Scholar]
- Validity of the montreal cognitive assessment for traumatic brain injury patients with intracranial haemorrhage. Brain Inj. 2013;27:394-8.
- [Google Scholar]
- MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards neuropsychological battery after TIA and stroke. Stroke. 2012;43:464-9.
- [Google Scholar]
- The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-9.
- [Google Scholar]
- Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92:519-30.
- [Google Scholar]
- Six-month functional recovery of stroke patients: A multi-time-point study. Int J Rehabil Res. 2015;38:173-80.
- [Google Scholar]
- Understanding the pattern of functional recovery after stroke: Facts and theories. Restor Neurol Neurosci. 2004;22:281-99.
- [Google Scholar]
- Motor recovery after stroke: A systematic review of the literature. Arch Phys Med Rehabil. 2002;83:1629-37.
- [Google Scholar]
- Functional outcome following rehabilitation in chronic severe traumatic brain injury patients: A prospective study. Ann Indian Acad Neurol. 2012;15:120-4.
- [Google Scholar]






