Translate this page into:
Coexistence of arteriovenous malformation with nonfunctioning pituitary adenoma
Address for correspondence: Dr. Murat Şakir EKŞI, Department of Orthopedic Surgery, University of California at San Francisco, 500 Parnassus Avenue, MU 320 West, San Francisco, CA 94143-0728, USA. Email: muratsakireksi@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Concomitant brain pathologies have been reported in the literature.[12] Cerebral aneurysms concomitant with pituitary adenomas have been previously reported.[23] However, arteriovenous malformations (AVM) concomitant with pituitary adenomas are very rare.[456] We present a patient, who has been successfully treated with gamma-knife radiosurgery (GKR) for AVM concomitant with nonfunctioning pituitary adenoma. We also discuss the case with the current literature. To our knowledge, this is the first patient who has been successfully treated with GKR for AVM concomitant with pituitary adenoma.
A 28-year-old female, who had cerebral AVM concomitant with pituitary adenoma, was referred to our GKR unit in 2006. At the time of referral, she had 5-time-embolized AVM in the left fronto-parietal lobe and residual pituitary adenoma from the surgery in 2001. Her neurological and ophthalmological examinations were intact. Her hormone levels were unremarkable. The pituitary adenoma was located in close proximity to the left internal carotid artery [Figure 1a]. GKR was planned for both pathologies. The doses of radiation for the pituitary adenoma and the AVM nidus were 18 Gy defined to the 50% isodose line and 22 Gy defined to the 50% isodose line, respectively [Figures 1a and 2a]. The patient was followed-up with brain magnetic resonance imaging (MRI) every 3 months in the 1st year and every 6 months in the following 2 years. At the end of the 3rd year, both brain MRI and brain digital subtraction angiography were performed. The pituitary adenoma had no progression while a residual AVM nidus was still present [Figures 1b and 2b]. We planned a second-stage GKR for the residual AVM. The dose of radiation for the AVM nidus was 20 Gy defined to the 50% isodose. The AVM nidus became obliterated 4.5 years after the second-stage GKR [Figure 2c]. The patient's pituitary hormone levels were unremarkable at the last follow-up. She had no new seizures under anti-epileptic drug regimens during the course of the treatment.
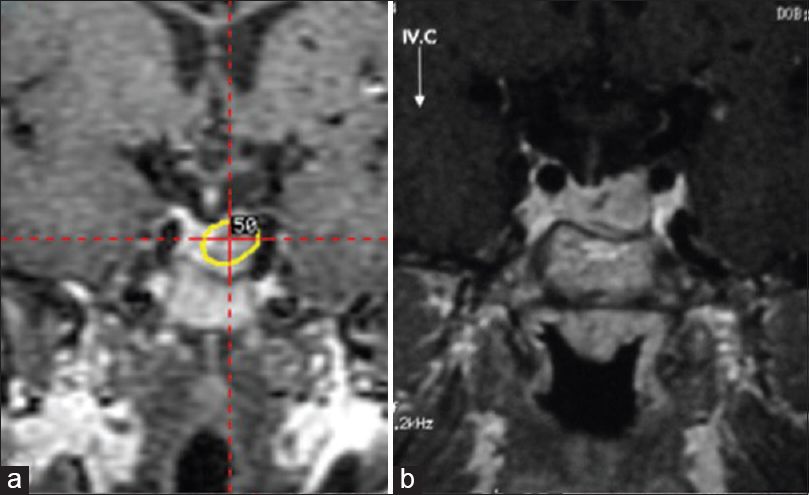
- Residual pituitary adenoma is located in close proximity to the left internal carotid artery (a) follow-up magnetic resonance imaging 3 years after the gamma-knife radiosurgery (b)

- Residual arteriovenous malformation is apparent in 2006 (a), nidus size diminished at the 3rd-year follow-up (b). Nidus has been obliterated 7.5 years after the first gamma-knife radiosurgery (c)
Cerebral vascular lesions may co-exist with brain tumors.[12] Prevalence of pituitary adenomas is 16.7%, whereas prevalence of AVMs is 18 in 100 000 adults.[78] Incidence of cerebral aneurysms in patients with brain tumors is 0.2–0.7% while the incidence of AVMs in the same patient population is only 0.1%.[9101112] Cerebral aneurysms were reported in 0.04–7.4% of patients with pituitary adenoma.[356] Since the first case reported by Licata et al. in 1986, only four cases have been reported to have AVM concomitant with pituitary adenoma [Table 1].[491314]

Meta-analyses depicted the high success rates of GKR in both AVMs and pituitary adenomas, particularly when the surgery is not the best choice of treatment for patients with complicated pathologies. It has been reported that 42.3–89% of nonfunctioning pituitary adenomas responded to GKR, functioning adenomas responded to GKR in different ranges based on the hormone (s) secreted by the ademonas (prolactinoma = 17.4–50%; acromegaly = 36.9–82%; Cushing's disease = 27.9–54%).[15] On the other hand, complete obliteration rate of cerebral AVMs is 96% (range = 0–100%) with microsurgery, 38% (range = 0–75%) with stereotactic radiosurgery, and 13% (range = 0–94%) with embolization.[16] Even though success rate of GKR in AVMs after embolization is lower than that of GKR alone (41% vs. 59%), complication rates of both approaches are somehow similar (hemorrhage = 7.3% vs. 5.6%; permanent neurological deficit = 3.3% vs. 3.4%).[17]
Previously, Licata et al. and Xu et al. reported the successful resection of both pituitary adenoma and AVM.[914] Their patients had had no previous treatments for pituitary adenoma and AVM. Furuya et al., reported a patient with spontaneous disappearance of AVM after hemorrhage.[4] They detected the AVM before the pituitary surgery. Then, they operated the patient using transsphenoidal route, after which left hemiparesis, right deafness, and nystagmus developed. They detected hematoma in the previous location of the dural AVM.[4] Remote hemorrhage of AVM after deep brain stimulation surgery has been reported in the literature.[18] In a meta-analysis about residual nonfunctioning pituitary adenomas, regrowth was detected in 12% patients with undetectable amount of residual adenoma while the incidence rises to 46% in detectable amounts of residual tumor.[19] Our patient had been operated for pituitary adenoma before she was referred to us. Her residual lesion was in close proximity to the left internal carotid artery. As she had been previously operated, the adenoma was suspected to have a fibrotic capsule. Her AVM had been previously embolized for 5 times. Due to multiple previous invasive attempts for both pathologies, and risk of hemorrhage during and/or after the pituitary surgery we planned GKR for both pathologies. The pituitary adenoma had no progression, and the AVM diminished in size after 3 years of follow-up. Our patient received a second-stage GKR for the AVM bed. She had no new neurologic deficits and/or other complications in follow-ups. The AVM nodule obliterated 7.5 years after the first GKR.
In conclusion, AVM concomitant with pituitary adenoma is a very rare condition. Treatment should be tailored to the patient, based on the location of AVM and the type of adenoma, previous treatment approaches, and related co-morbidities. To our knowledge, this is the first case of AVM concomitant with nonfunctioning pituitary adenoma both treated successfully with GKR.
Acknowledgments
Murat Şakir Ekşi, M.D. was supported by a grant from Tubitak (The Scientific and Technological Research Council of Turkey), Grant number: 1059B191400255. We thank to Selim Olduz for his technical support to the manuscript.
References
- Two different primary tumors in the same brain - A case report and review of the literature. J Neurol Sci Turk. 2014;31:635-40.
- [Google Scholar]
- MRI finding of simultaneous coexistence of growth hormone-secreting pituitary adenoma with intracranial meningioma and carotid artery aneurysms: Report of a case. Pituitary. 2007;10:299-305.
- [Google Scholar]
- Association between pituitary adenomas and intracranial aneurysms: An illustrative case and review of the literature. Neurol India. 2007;55:410-2.
- [Google Scholar]
- Brain stem haemorrhage during transsphenoidal surgery. Possible risk of the manoeuvre of increasing the endexpiratory pressure in a case of pituitary adenoma associated with dural arteriovenous malformation. Case report. Acta Neurochir (Wien). 1993;125:188-91.
- [Google Scholar]
- Rupture of anterior communicating artery aneurysm during transsphenoidal surgery for pituitary adenoma. Surg Neurol. 1983;20:67-70.
- [Google Scholar]
- Association of cerebral aneurysm with pituitary adenoma. Surg Neurol. 1979;12:503-7.
- [Google Scholar]
- A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001;124:1900-26.
- [Google Scholar]
- Management of associated primary cerebral neoplasms and vascular malformations: 2. Intracranial arterio-venous malformations. Acta Neurochir (Wien). 1986;83:38-46.
- [Google Scholar]
- Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien). 1972;27:189-204.
- [Google Scholar]
- Delayed postoperative hemorrhage from intracranial aneurysm after craniotomy for tumor. Neurology. 1961;11:225-31.
- [Google Scholar]
- Pituitary adenoma associated with dural arteriovenous fistula. Case report. Acta Neurol (Napoli). 1993;15:442-8.
- [Google Scholar]
- Association of pituitary adenoma with intracranial arteriovenous malformation. Chin Med J (Engl). 1996;109:734-6.
- [Google Scholar]
- Stereotactic radiosurgery/radiotherapy for pituitary adenomas: A review of recent literature. Neurol Med Chir (Tokyo). 2010;50:749-55.
- [Google Scholar]
- Treatment of brain arteriovenous malformations: A systematic review and meta-analysis. JAMA. 2011;306:2011-9.
- [Google Scholar]
- Stereotactic radiosurgery with and without embolization for intracranial arteriovenous malformations: A systematic review and meta-analysis. Neurosurg Focus. 2014;37:E16.
- [Google Scholar]
- Fatal hemorrhage from AVM after DBS surgery: Case report. Neuromodulation. 2013;16:414-7.
- [Google Scholar]
- Natural history of postoperative nonfunctioning pituitary adenomas: A systematic review and meta-analysis. Neuroendocrinology. 2012;96:333-42.
- [Google Scholar]





