Translate this page into:
Clinico-radiological profile and outcome of dengue patients with central nervous system manifestations: A case series in an Eastern India tertiary care hospital
Address for correspondence: Dr. Souren Pal, Department of General Medicine, Nil Ratan Sircar Medical College and Hospital, Kolkata - 700 014, West Bengal, India. E-mail: drsourenpal@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objective:
Dengue, an acute viral disease, transmitted by Aedes mosquitoes, has a variable clinical spectrum ranging from asymptomatic infection to life-threatening dengue hemorrhagic fever and dengue shock syndrome. However, neurological complications, in general, are unusual but have been observed more frequently in the recent past, and some studies highlighted varied neurological complications during the course of illness. Although dengue is classically considered a nonneurotropic virus, there is increasing evidence for dengue viral neurotropism. In this study, we have evaluated clinico-radiological profile and outcome of nine serologically confirmed dengue patients having varied manifestations of central nervous system (CNS) involvement.
Materials and Methods:
All the consecutive patients presented with neurological complications with positive serology for dengue infection (IgM positivity) in Department of Medicine, in a tertiary care hospital in Eastern India from August 2013 to October 2014 were included in the study. These patients were subjected to a detailed clinical evaluation, laboratory assessment including complete hemogram, coagulation profile, liver function test, serum electrolytes, and routine CSF (Cerebrospinal Fluid) study with the exclusion of other common neuroinvasive pathogens.
Results:
Out of 9 patients with neurological complications associated with confirmed dengue infection, 2 (22%) patients had dengue encephalopathy, 5 (56%) patients have dengue encephalitis, 1 (11%) patient had dengue meningitis, and 1 (11%) patient had postdengue immune-mediated CNS involvement.
Conclusion:
This case series reaffirms the occurrence of varied CNS manifestations in dengue virus infection and underlines the importance of inclusion of dengue in the differential diagnosis of acute encephalitis syndrome.
Keywords
Acute disseminated encephalomyelitis
dengue
encephalitis
facial nerve
meningitis
meningoencephalitis
meningoencephalomyelitis
Introduction
Clinical manifestations of dengue virus infection have been classified according to severity by the World Health Organization (WHO) as: Nonspecific febrile illness, classical dengue syndrome, dengue hemorrhagic fever (DHF), and dengue shock syndrome.[1] In 2009, WHO adjustments in the classification of the disease resulted in the recognition of two main presentations of dengue. These are referred to as dengue fever ± warning signs and severe dengue. Neurological dengue is classified as a form of severe dengue.[2] But, no standardized case definitions or diagnostic criteria for dengue encephalitis or encephalopathy have been agreed. Recent evidence from different studies suggests that the incidence of dengue encephalitis ranges from 4.2% to 13% of central nervous system (CNS) infections.[34]
Materials and Methods
In this hospital-based case series in a tertiary care hospital in Eastern India, all the consecutive serologically confirmed dengue patients (positive IgM dengue antibody) of either sex above 12 years of age with neurological complications, including impaired consciousness (Glasgow Coma Scale or GCS Score ≤14) or neck stiffness or focal neurological signs or seizure admitted in the Department of Medicine from August 2013 to October 2014, were included in the series. Patients meeting the diagnostic criteria of multiple neuroinvasive pathogens in addition to dengue and patients having comorbid conditions that have an effect on CNS were excluded from the series. The dengue was diagnosed on the basis of the positive serum IgM antibody to dengue virus by the IgM antibody capture enzyme-linked immunosorbent assay (MAC ELISA) method with NOVATEC Kit. It was a qualitative assay and titers were not measured. The baseline characteristics, including age, sex, occupation, residence (rural/urban), were noted. After taking detailed clinical history, clinical evaluation, including detailed neurological examinations, was done. The laboratory investigations, including complete hemogram, liver function test, renal function test, serum electrolytes, coagulation profile, malaria parasite dual antigen (MPDA) for Plasmodium vivax, and Plasmodium falciparum by immunochromatographic assay of both plasmodium lactate dehydrogenase (pLDH) and histidine-rich protein-2 (HRP-2) and ELISA for human immunodeficiency virus were done. Chest X-ray and electrocardiography were done in all patients. The routine cerebrospinal fluid (CSF) analysis and special investigations in CSF for Japanese encephalitis (JE) IgM, herpes simplex virus polymerase chain reaction (HSV PCR), Chikungunya IgM, and dengue IgM were done in all patients. In one case, anti-nuclear antibody (ANA), perinuclear anti-neutrophil cytoplasmic antibodies (pANCA), cytoplasmic anti-neutrophil cytoplasmic antibodies (cANCA) were done to exclude autoimmune disease. 1.5 Tesla magnetic resonance imaging (MRI) scan of the brain was also done in all patients.
With the view of these clinicopathological and radiological profiles, patients were classified as dengue encephalitis by presence of fever, acute signs of CNS involvement within 7 days of onset of fever, CSF pleocytosis without other neuroinvasive pathogen, reactive IgM dengue antibody in serum and/or CSF, dengue meningitis by fever, acute signs of CNS involvement or presence of focal neurological signs restricted to meninges only without involvement brain parenchyma, CSF pleocytosis without other neuroinvasive pathogen, reactive IgM dengue antibody in serum and/or CSF, dengue encephalopathy by signs CNS involvement, normal CSF study, reactive IgM dengue antibody in serum, and presence of dengue-associated complications such as hepatic failure, metabolic acidosis, hyponatremia, prolonged shock, disseminated intravascular coagulation, brain hemorrhage, postdengue immune-mediated CNS involvement by presence of signs, CNS involvement that appear in 2nd week of febrile initiation, CSF pleocytosis without other neuroinvasive pathogen, and reactive IgM dengue antibody only in serum.
The outcome was evaluated at the end of 1-month whether the recovery was partial (remain dependent for daily living activities) or complete (remain independent for daily living activities).
Results
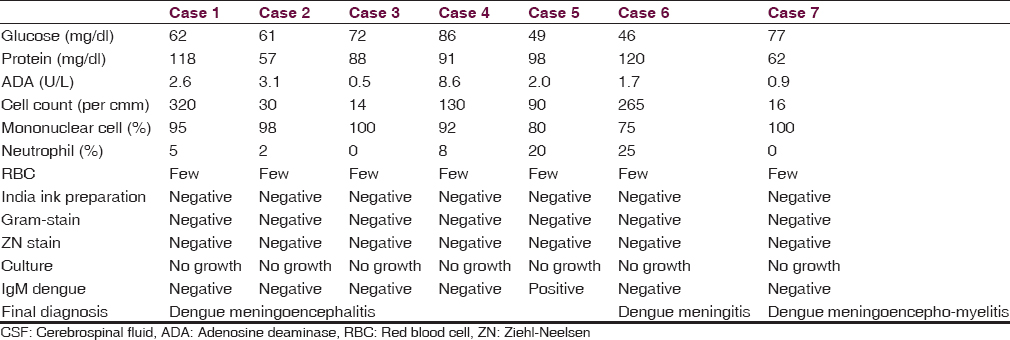
Nine patients of serologically confirmed dengue infection with CNS manifestations were observed during this period. Among them, 2 (22%) patients had dengue encephalopathy, 5 (56%) patients have dengue encephalitis, 1 patient (11%) had dengue meningitis, and 1 (11%) patient had postdengue immune-mediated CNS involvement [Figure 1]. The age of the patients ranged from 12 to 45 years with mean age of 28.9 ± 12.1 years. Male and female ratio was 2:1 and rural and urban population ratio was 2:1. Fever, headache, and vomiting were present in all patients. Average number of days between onset of fever to disorientation were 4.3 ± 1.9 days [Figure 2]. Generalized tonic-clonic seizure (GTCS) convulsion was present in four patients (44%). Cranial nerve palsy was present in 1 patient (11%). Median GCS Score on admission was 9 (range 7–15) [Figure 2]. CSF abnormality was not detected in two patients of dengue encephalopathy. Rest of the seven patients had elevated CSF cell count (range 14–320 per cmm with average 124 per cmm) [Figure 2] with predominant lymphocytosis, elevated CSF protein content (90 ± 24 mg/dl) [Figure 2], and normal glucose level. IgM dengue antibody in CSF was positive in one case of dengue encephalitis out of 5 patient of dengue encephalitis and 1 patient of dengue meningitis, altogether 17%. Median platelet count on admission was 2 lac/cmm with range 1–2.5 lac/cmm [Figure 2]. Two out of nine patients (22%) had thrombocytopenia. Purpura was present in two patients (22%), one of dengue encephalitis and other dengue encephalopathy. Both the patients of dengue encephalopathy had hyponatremia with elevated transaminase and one patient in addition to that had neutropenia and thrombocytopenia. In all patients in this series, they had a complete recovery at the end of 1-month.
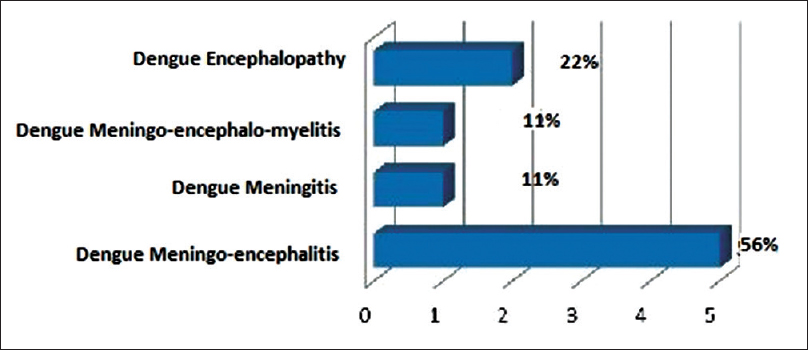
- Distribution of central nervous system manifestation in dengue infection
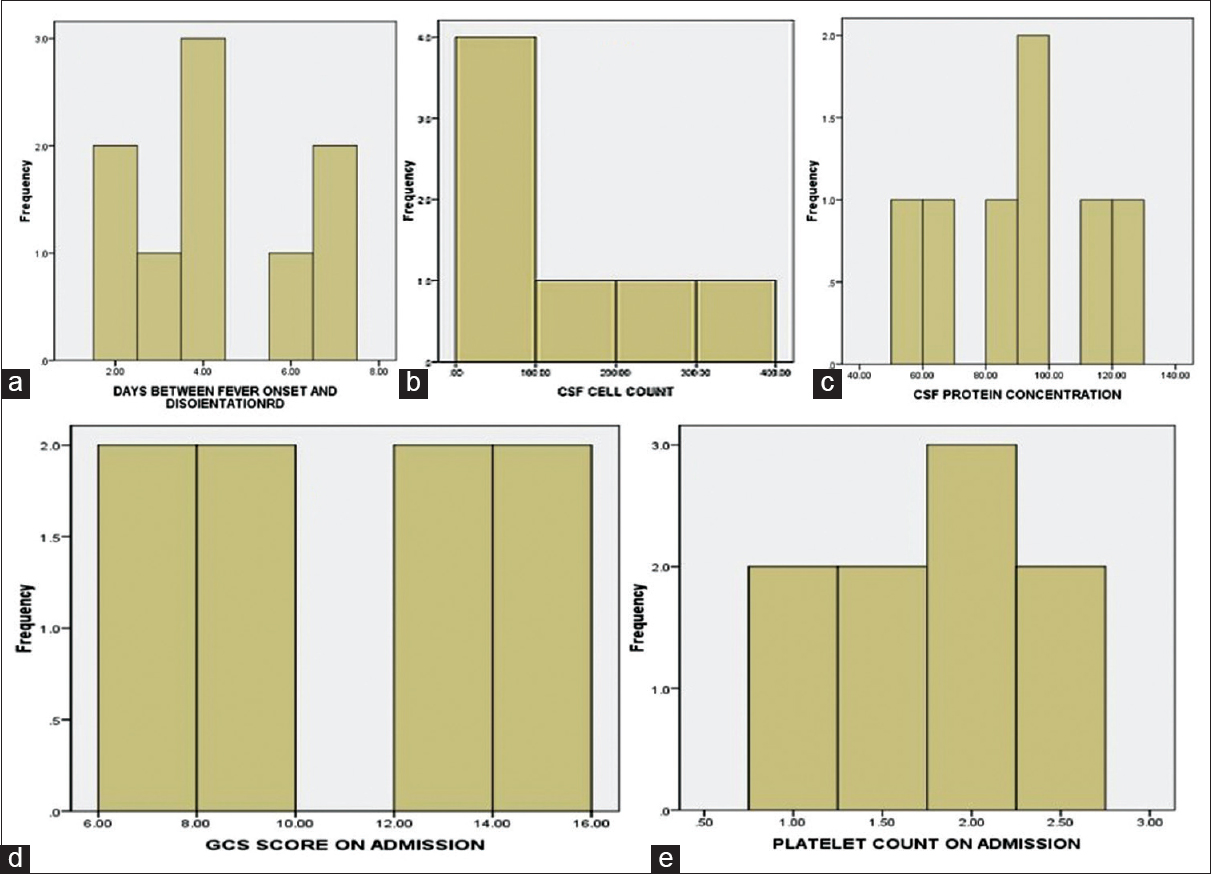
- Histogram showing (a) number of days between fever onset and disorientation. (b) Cerebrospinal fluid cell count. (c) Cerebrospinal fluid protein concentration. (d) Glasgow Coma Scale score on admission. (e) Platelet count on admission
Case illustration
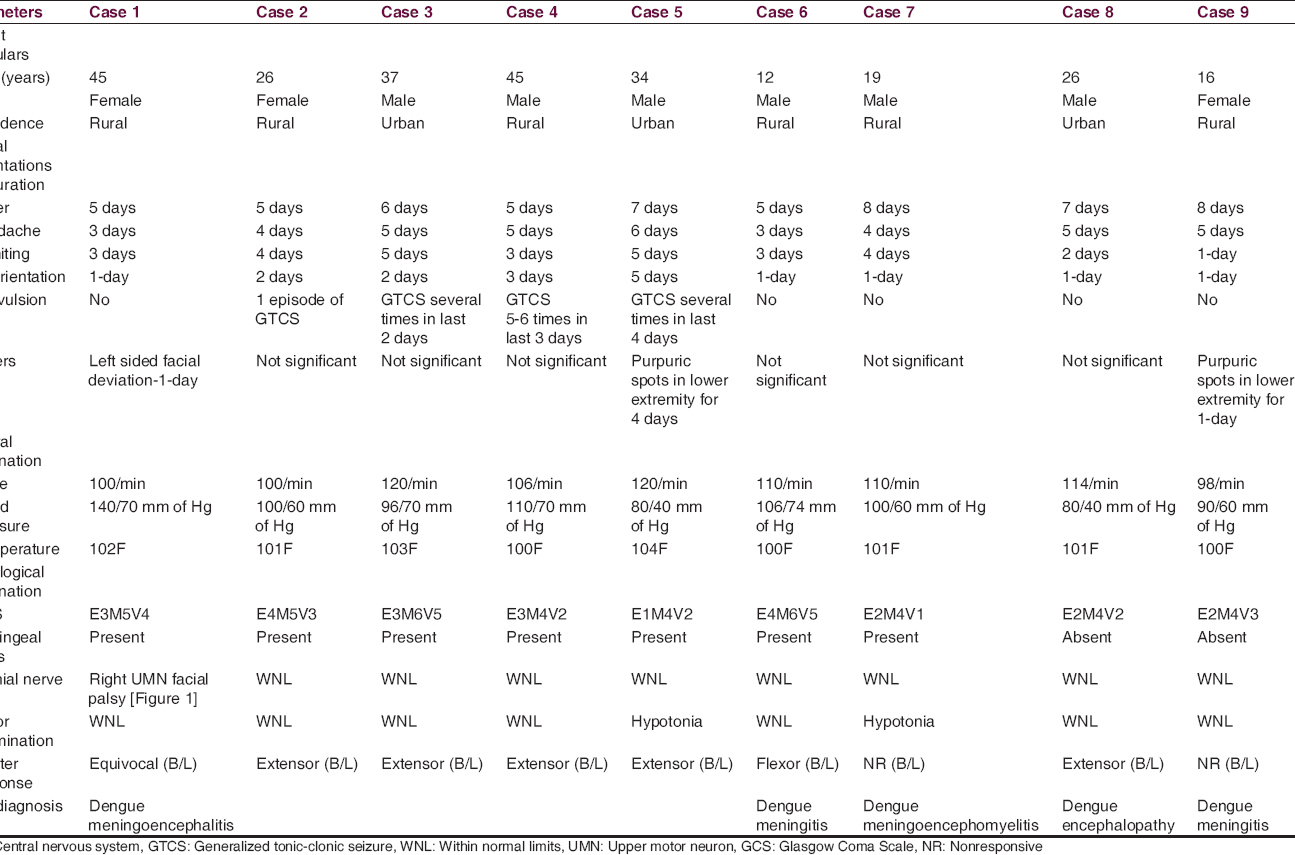
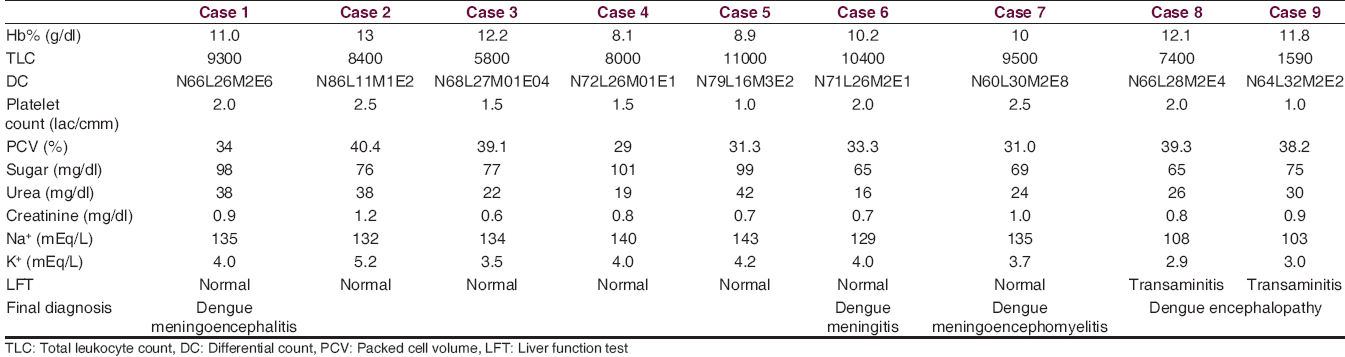
In all these cases, after clinical evaluation [Table 1], we have done routine blood tests [Table 2], routine CSF examinations [Table 3], MPDA for P. vivax and P. falciparum by immunochromatographic assay of pLDH and HRP-2 (both), serum dengue IgM by MAC ELISA method with NOVATEC Kit, MRI of brain (1.5 Tesla), and special investigations in CSF for JE IgM, HSV PCR, Chikungunya IgM and Dengue IgM and in one case we have done autoimmune panel. In all of the patients MPDA was negative, dengue IgM was positive in serum and the CSF failed to show the presence of JE IgM, Chikungunya IgM, and HSV by PCR. None of the patients in this series had other comorbid conditions, and none was alcoholic or smoker.
Clinical presentation, MRI findings and treatment courses are briefly described as follows.
Patients of dengue meningoencephalitis
Case report 1
A 45-year-old female patient presented with high-grade continuous fever for 5 days, headache and vomiting for 3 days, disorientation for 1-day, and facial deviation to the left side for 1-day.
On neurological examination, she had neck rigidity with positive Kernig sign. GCS Score was E3M5V4. Right-sided upper motor neuron (UMN) type facial nerve palsy was noted [Figure 3, Right panel]. Motor function, sensory function, deep tendon reflexes, cerebellar function, and autonomic function were all within normal limit. Planter was equivocal in both sides.
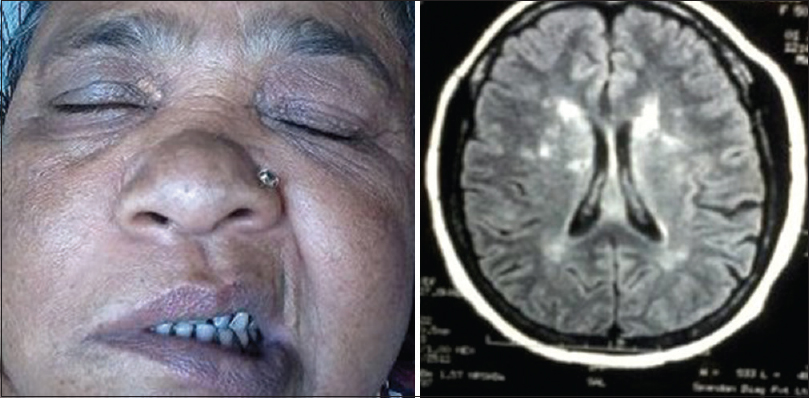
- Right: Right-sided upper motor neuron type facial nerve palsy, Left: Magnetic resonance imaging of brain in T2 fluid attenuation inversion recovery sequence showing multiple tiny nonenhancings ischemic demyelination in lentiform nucleus, peri- and para- ventricular white matter and centrum semiovale on both sides
MRI scan of brain (plain and contrast) done on 6th day of fever, showed multiple tiny nonenhancing hypo on T1 and hyperintense lesions on T2, T2 fluid attenuation inversion recovery (FLAIR) sequence suggestive of ischemic demyelination in lentiform nucleus, peri- and para-ventricular white matter and centrum semiovale on both sides [Figure 3, Left panel]. So, a diagnosis of dengue meningoencephalitis with right sided UMN type facial palsy was made.
The patient was treated with intravenous (IV) fluid and antipyretics, and at the end of the 2nd week patient became completely afebrile with no neck rigidity but facial palsy persisted partially. Patient was discharged and on follow-up at the end of the 4th week there was complete resolution of facial palsy.
Case report 2
A 26-year-old girl presented with high-grade continuous fever associated with chills and rigor for 5 days, severe headache anorexia, nausea, multiple episodes of vomiting for 4 days, and disorientation for 2 days. There was a history of one episode of GTCS convulsion day before admission. There was no similar illness in the past.
On neurological examination, she had neck rigidity with positive Kernig sign. GCS Score was E4M5V3. No cranial nerve abnormality was noted. Motor function, sensory function, reflexes, cerebellar function, and autonomic function were all within normal limit. Planter was bilateral extensor.
MRI scan of brain (plain and contrast) done on 6th day of fever, showed mild diffusion restricted nonenhancing altered signal intensity (hypointense in T1, hyperintense in T2 and FLAIR) in both thalamic and left medial temporal lobe suggestive of encephalitis [Figure 4]. The patient was diagnosed as dengue meningoencephalitis.
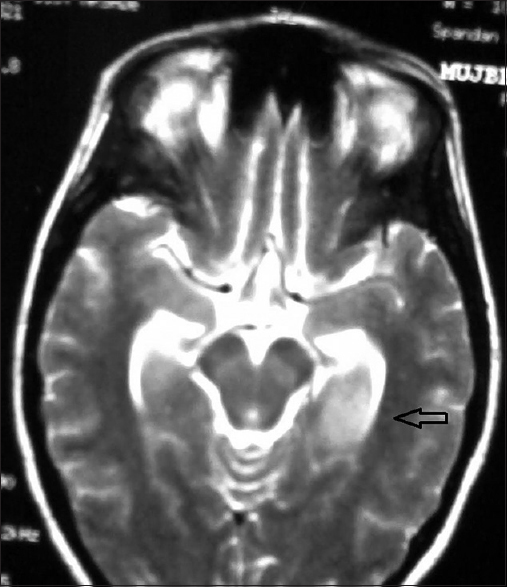
- Magnetic resonance imaging scan of brain in T2 sequence showed non-enhancing altered signal intensity in both thalamic and left medial temporal lobe suggestive of encephalitis
Patient was treated only with IV fluid and paracetamol as antipyretic. Patient improved gradually with normal sensorium gained on the 10th day of hospital stay with no neck rigidity. He had uneventful hospital stay thereafter and was discharged after 2 weeks.
Case report 3
A 37-year-old man presented with high-grade continuous fever associated with chills and rigor for 6 days, severe headache, multiple episodes of vomiting for 5 days, and disorientation for 2 days. There was a history of repeated GTCS in last 2 days. There was no similar illness in the past. He was drowsy but arousable on examination (GCS E3M6V5).
On neurological examination, he had only neck rigidity with positive Kernig sign and planter was bilateral extensor. Rest of the CNS examination was within normal limit. Other systems were also within normal limit.
MRI of brain, done on 7th day of fever, showed diffuse brain edema without any structural abnormality, and the patient was diagnosed as dengue meningoencephalitis based on clinical presentation, MAC ELISA reactivity of IgM dengue, CSF profile, and normal routine blood test during and after GTCS.
Patient was treated only with IV fluid, paracetamol as antipyretic and anticonvulsant (IV phenytoin). On 3rd day onward he had no focal or generalized convulsion. Patient improved gradually, and neck rigidity was absent on the 5th day of hospital stay. Thereafter he had an uneventful hospital stay and was discharged on the 14th day.
Case report 4
A 45-year-old male patient, presented with high-grade continuous fever for 5 days without chills and rigor, persistent headache for 5 days, recurrent episodes of vomiting, and GTCS followed by drowsiness for last 3 days.
He was semiconscious (GCS E3M4V2) on first examination, and neurological examination showed he had only neck rigidity with positive Kernig sign. Deep tendon reflexes are all absent and planter was bilaterally extensor. Rest of the CNS examination could not be done. Other systems were also within normal limit.
MRI brain (plain and contrast), done on the 6th day of fever, showed bilateral symmetrical T2 hyperintensities in both thalamic regions [Figure 5]. So, the diagnosis of dengue meningoencephalitis was done.
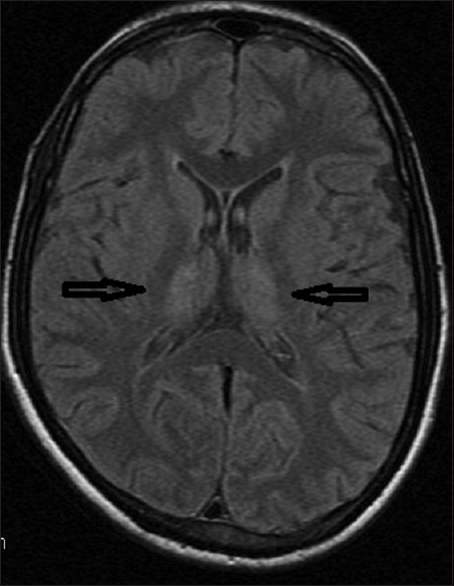
- Magnetic resonance imaging brain in T2 fluid attenuation inversion recovery sequence showed bilateral symmetrical hyperintensities in both thalamic regions
This patient also was treated only with IV fluid, paracetamol as antipyretic and anticonvulsant. On 2nd day onward he had no event focal or generalized convulsion. Patient improved gradually, and neck rigidity was absent on the 11th day of hospital stay. Patient had uneventful hospital stay in the rest of the days and was discharged on the 14th day.
Case report 5
A 34-year-old male patient presented with high-grade continuous fever associated with chills and rigor for 7 days, persistent severe headache for 6 days, multiple episodes of vomiting, and disorientation for 5 days. There was a history of multiple episodes of GTCS for last 4 days. He had also purpuric spots in lower extremities for last 4 days.
On neurological examination, she had neck rigidity with positive Kernig sign. GCS Score was E1M4V2. No cranial nerve abnormality was noted. Motor examination revealed hypotonia on all four limbs. Planter was bilateral extensor. Rest of the CNS examination could not be done. Other systems were also within normal limit.
MRI scan of brain (plain and contrast), done on 8th day of fever, showed mild diffusion restricted altered signal intensity (hypointense in T1, hyperintense in T2, and FLAIR) in both thalamic and splenium of corpus callosum region [Figure 6], and the diagnosis of dengue meningoencephalitis was done.
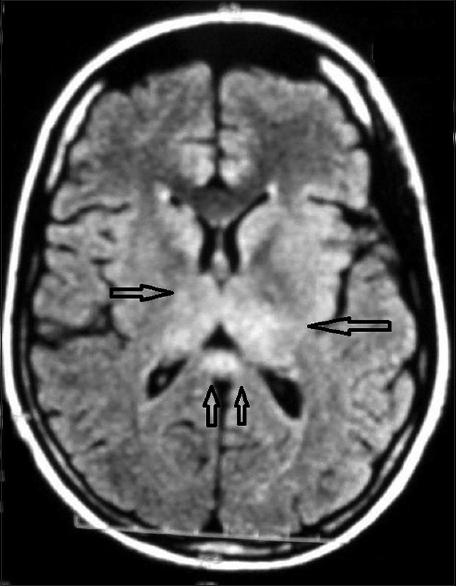
- Magnetic resonance imaging scan of brain T2 fluid attenuation inversion recovery sequence showed altered signal intensity in both thalamic and splenium of corpus callosum region
Again, treatment was started only with IV fluid, paracetamol as antipyretic and anticonvulsant. Patient had several episodes of GTCS till 7th day of hospital admission. Then it gradually decreased and 12th day onward he had no episode of GTCS. Neck rigidity was absent on 16th day. Thereafter he had an uneventful hospital stay and was discharged on 21st day.
He was the only patient to have IgM dengue positivity in CSF.
Patient of dengue meningitis
Case report 6
A 12-year-old boy presented with 5 days continuous high-grade fever with chills and rigor, severe headache, anorexia, nausea, vomiting for 3 days, neck stiffness for 2 days, and drowsiness for 1-day. However, there was no history of loss of consciousness and convulsion.
His GCS was E4M6V5 on admission. On neurological examination, he had only neck rigidity with positive Kernig sign. Rest of the CNS examination was within normal limit. Other systems were also within normal limit.
MRI of the brain done on the 6th day of fever showed no detectable abnormality. A diagnosis of dengue meningitis was made based on the clinical presentation that is, fever and neck rigidity, MAC ELISA positivity for dengue, and the suggestive CSF profile.
His clinical condition improved gradually on conservative management with IV fluids and paracetamol. Neck rigidity disappeared after 10 days. He was discharged from the hospital after 2 weeks of hospital stay in a stable condition.
Patient of post dengue immune-mediated central nervous system abnormality
Case report 7
A 19-year-old male patient presented with high-grade continuous fever, headache, vomiting for 8 days, severe headache and vomiting for 4 days, and altered sensorium for 1-day. There was no history of convulsion during this period.
On neurological examination, he had neck rigidity with positive Kernig sign. GCS Score was E2M4V1. No cranial nerve abnormality was noted. Plantar was nonresponsive bilaterally, and hypotonia was found in all four limbs. Power could not be tested.
MRI scan of brain done on 9th day of fever showed areas of hyperintensities involving bilateral periventricular and parietal region with signal changes involving right cerebellar peduncle with involvement of upper brainstem which were suggestive of acute disseminated encephalomyelitis (ADEM) [Figure 7].
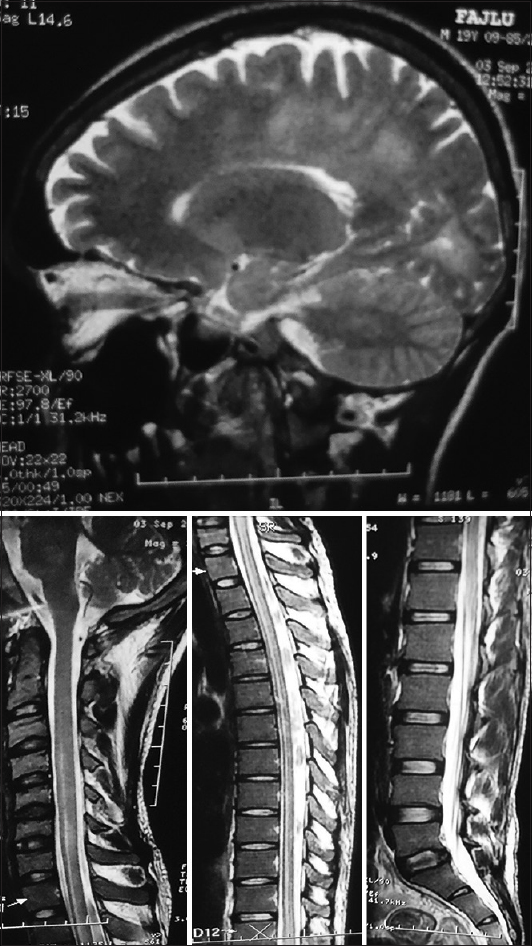
- Upper: Magnetic resonance imaging of the brain in T2 sequence showing areas of hyperintensities involving bilateral periventricular and parietal region with signal changes involving right cerebellar peduncle with the involvement of upper brainstem. Lower Right, Middle, Left: Magnetic resonance imaging of spinal cord in T2 sequence showing hyperintense intramedullary signal changes involving spinal cord extending from C3 level downward including entire cervical (Lower Right Panel), thoracic (Lower Middle Panel), lumbar region until conus (Lower Left Panel) suggestive of myelitis with cord edema
Patient became fully conscious after 2 days. Then on motor system examination it was revealed that he had quadriparesis with the power of right lower extremity 1/5, left lower extremity 2/5, and both right and left upper extremity 4/5. All deep tendon reflexes were diminished. There was no sensory impairment. MRI of whole spine showed hyperintense intramedullary signal changes involving spinal cord extending from C3 level downward until conus suggestive of myelitis with cord edema [Figure 7]. CSF study showed immunoglobulin synthesis index 0.72, immunoglobulin synthesis rate 6.5 mg/day with no oligoclonal bands. VEP was also normal. Autoimmune markers such as ANA, pANCA, and cANCA were negative.
So, a revised diagnosis of dengue meningoencephalomyelitis was made, and he was put on IV methylprednisolone 1 g once daily for 5 days. Patient regained his power in upper extremities, but both lower extremities had 4/5 power on discharge. At first follow-up, after 3 weeks of discharge he had no residual paraparesis.
Patients of dengue encephalopathy
Case report 8 and 9
Both the patient presented with fever (for 7 and 8 days), headache (both for 5 days), recurrent episode of vomiting (for 2 days and 1-day), and disorientation (both for 1-day). One had purpuric spots in lower extremities for 1-day. They had impaired sensorium on admission (GCS 8 and 9) but unremarkable neurological examination except for abnormal plantar response (B/L extensor and nonresponsive). One patient had severe hyponatremia, and other one had hyponatremia, neutropenia, and thrombocytopenia. Both the patients had elevated transaminase level. Both the patients had normal MRI brain and normal CSF picture. Both the patients regained their sensorium after conservative management and electrolyte correction within 7 days. So, metabolic abnormalities caused by dengue virus were considered as the cause for the CNS manifestations in both the patients and diagnosed as dengue encephalopathy. Both of them were discharged at the end of 2nd week.
Discussion
Dengue virus is a member of the Flavivirus genus of the family Flaviviridae. There are four genetically and antigenically distinct serotypes of dengue virus (DENV 1–4).
The incidence of dengue infection in patients with suspected CNS infection is noted to range from 4.2% in Southern Vietnam to 13.5% in Jamaica.[34] The higher frequency of neurological dengue (13.5%) reported in Jamaica may be attributed to the inclusion of both adults and children in the study. Neurological manifestations of dengue noted in the Jamaican study include; meningitis (34%), seizures (11%), acute flaccid paralysis, and Guillain–Barré syndrome (4%).[3]
In the present case series, varied CNS manifestations were observed in association with dengue fever. Demographically, patients belonged to different ages; males outnumbered females. Various studies mentioned age range 18–35 years (mean age 27 years), 5–65 years, 11–60 years, and male sex predominance in a large cohort related to neurological complications associated with dengue fever.[56]
Patients with dengue encephalitis may be at risk of developing complications of DHF although some patients with encephalitis may not show any characteristic features of the disease.[47] Two patients in this series had features of hemorrhagic fever; one was diagnosed as dengue meningoencephalitis (case report 5), and other one was dengue encephalopathy (case report 9).
Neurological features of dengue infection was categorized by Carod-Artal et al. in their study into: (1) Dengue CNS involvement (dengue encephalopathy, dengue encephalitis, immune-mediated syndromes that is, transverse myelitis, ADEM, mononeuropathies, cranial nerve palsies, Guillain–Barre syndrome, and other or nonspecified dengue CNS involvement), (2) dengue-associated neuromuscular complications (Guillain–Barre syndrome, rhabdomyolysis, other or nonspecific peripheral neuromuscular complication), and (3) dengue-associated neuro-ophthalmic complications.[8]
The same was grouped into three categories by Murthy as: (I) Related to neurotrophic effect of the virus: Encephalitis, meningitis, myositis, rhabdomyolysis, and myelitis; (II) related to the systemic complications of dengue infection: Encephalopathy, stroke (both hemorrhagic and ischemic), hypokalemic paralysis, and papilledema; and (III) postinfection: ADEM, encephalomyelitis, myelitis, neuromyelitis optica, optic neuritis, Guillain–Barré syndrome, probable Miller–Fisher syndrome, mononeuropathies such as phrenic neuropathy, long thoracic neuropathy oculomotor palsy, isolated Bell's palsy, abducens nerve palsy, maculopathy, and fatigue syndrome. Postinfection neurological syndromes are usually immune mediated.[9]
Spinal cord involvement in the form of transverse myelitis can happen in patients with dengue both during and after infection. Direct virus invasion can take place in the parainfectious stage whereas immune-mediated factors are noted postinfection.[1011] Postinfectious immune-mediated myelitis usually arises 1–2 weeks after the onset of initial symptoms whereas parainfectious myelitis can take place within the 1st week of infection.[1011] Some cases of ADEM have been reported in the convalescence stage of dengue virus infection.[12] The 7th patient in this series became disoriented 7 days after onset of fever and quadriplegia were discovered on the 11th day of onset of fever. MRI scan of the brain and spinal cord were done on 9th and 11th day, respectively, and showed features suggestive of meningoencephalomyelitis. Considering the time of onset of neurological manifestations and response to IV methyl prednisolone, stamping the patient as postdengue immune-mediated encephalomyelitis were justifiable.
The involvement of the CNS has always been thought to be secondary to vasculitis and leaky capillary syndrome with resultant fluid extravasations, cerebral edema or hypoperfusion, prolonged shock, cerebral anoxia, metabolic disturbances such as hyponatremia, systemic and cerebral hemorrhages, liver failure, and/or renal failure. As such, it is usually called dengue encephalopathy. CSF analyses, including measurements of protein, glucose, and cell count, are usually normal in Dengue encephalopathy.[8] However, reports of virus isolation from brain tissue and CSF of patients with neurological symptoms suggest a direct virus invasion of the CNS in many of the cases. Although encephalopathy can exist in severe dengue as a result of multi-organ involvement, there is a growing body of evidence in support of dengue neurotropism.[41314]
Studies with mice models have shown that the IV inoculation of dengue induced cytotoxic factor is associated with transient compromise in the integrity of the blood brain barrier allowing leakage of protein and erythrocytes.[15] Infiltration by DENV infected macrophages has also been proposed as one of the mechanisms of direct CNS invasion.[16]
Neurological manifestations have been documented to occur with all dengue serotypes.[4] But, it is more frequent in serotype 2 and 3.[4]
The study by Carod-Artal et al. published in The Lancet in 2013 proposed a panel in which dengue encephalitis was defined by the following criteria: (1) Dengue CNS involvement, AND (2) presence of dengue virus RNA, IgM, or NS1 antigen in CSF, AND (3) CSF pleocytosis without other neuroinvasive pathogens. In the same article, they defined dengue encephalopathy by the following criteria: (1) Dengue CNS involvement, AND (2) presence of one of the following dengue-associated complications: Hepatic failure, metabolic acidosis, severe hyponatraemia, prolonged shock, disseminated intravascular coagulation, or brain hemorrhage, AND (3) normal CSF (in brain hemorrhage, blood in CSF is possible).[8]
But, the detection of specific IgM antibodies in CSF had high specificity but low sensitivity. Various published literature showed that the dengue IgM detection by ELISA in CSF had a high specificity (97–100%) but the sensitivity varied between 0% and 73%, depending on the method used.[17] This marker may confirm but its absence does not exclude the neurological manifestations associated with dengue. In general, these antibodies are not detected before the 7th day of the infection onset. Although, the PCR is usually positive for dengue virus at the first 5 days of infection in serum and/or CSF.[18]
Considering these data, the following diagnostic criteria has been suggested by Cristiane and Marzia for the definition of dengue encephalitis.[18]
-
Presence of fever
-
Acute signs of cerebral involvement such as altered consciousness or personality and/or seizures and/or focal neurological signs
-
Reactive IgM dengue antibody, NS1 antigen or positive dengue PCR on serum, and/or CSF. The choice of one of these laboratorial methods should be performed according to the time of infection onset
-
Exclusion of other causes of viral encephalitis and encephalopathy.
Our patient number 1–5 fulfilled the diagnostic criteria of dengue encephalitis as suggested by Cristiane and Marzia.[18] The presence of CSF abnormality and absence of hepatic failure, metabolic acidosis, severe hyponatraemia, prolonged shock, disseminated intravascular coagulation, or brain hemorrhage etc., indicate a direct CNS involvement by the dengue virus itself although the clinical presentations of these five patients were different. Their clinical features, CSF abnormality, and abnormalities in MRI of brain occurred within first 7 days of onset of fever which were probably not immune-mediated as it usually occur after 7 days of onset of clinical symptoms or in convalescence stage.
In this case series, varied lesions on MRI scan of the brain were revealed. Thalamic region was the only involvement in one patient (case report 4) but the other patients as described in case report 2 and 5 had involvement of medial temporal lobe and splenium of corpus callosum, respectively, in addition to thalamic involvement. There is a diagnostic significance of thalamic and basal ganglia lesions in JE, particularly in endemic areas during the postmonsoon period.[1920] But, these three patients’ serum and CSF were negative for JE. Thalamic involvement in dengue encephalitis were also been reported earlier by Bhoi et al. in three out of nine patients in a study of cranial imaging findings in 21 serologically confirmed dengue virus infection.[21] Kamble et al. have also described a case of dengue featuring JE like the bilateral thalamic involvement.[22] MRI of the brain with the involvement of splenium of corpus callosum having the appearance of dot (dot sign) have also been described in dengue encephalitis.[23]
Thalamic lesions are seen in a multitude of disorders, including vascular diseases, metabolic disorders, inflammatory diseases, trauma, tumor, and infections. Transient signal changes in pulvinar of the thalamus, as well as persistent thalamus changes following status epilepticus, has also been reported.[24] Again, basal ganglia and thalamus are vulnerable to hypoxia and ischemia and may be responsible for transient involvement as reported by Kamble et al.[22] In this series, no repeat MRI brain was done, which might have enlightened the mechanism of thalamic and basal ganglia involvement in dengue. The role of thalamus, in generalized seizure that appears simultaneously throughout the entire cortex, whether serve to amplify the rhythmic cortical discharge by involvement of thalamocortical circuit or to initiate the spike-and-wave discharge as evident from experimental work in animal models of generalized epilepsy and clinical data in human with idiopathic generalized epilepsy are still matter of debate.[2526]
Bhoi et al. opined that abnormal MRI lesions of brain in dengue are usually secondary to hypoxia or ischemia and is not related to hematological and biochemical changes or outcome as the outcome of their patients with or without MRI abnormality was not different.[21] And so, sole abnormality in cranial imaging study was not considered as diagnostic of dengue encephalitis. In this present series, also findings of MRI of the brain had no impact on the outcome.
The most interesting finding that come out from our case series is CNS involvement in dengue is not as uncommon as it is thought to be and the same might be more common in this part of the country with high endemicity of dengue – so is the importance of considering dengue as an etiology in patients presenting with features of acute encephalitis syndrome.
Dengue diagnostic test, which is followed in our institution is ELISA for dengue IgM from single serum sample and positive result of which is “highly suggestive” for active dengue infection according to WHO. This is important in a suspected outbreak in an endemic area when rapidity and specificity are more important than test sensitivity. But, results are preferably confirmed by either of PCR, viral culture, IgM seroconversion in paired sera, IgG seroconversion in paired sera, or four-fold IgG titer increase in paired sera.[2]
The limitations of our study are dengue confirmatory diagnostic tests could not be done due to nonavailability in the setting we work in and CSF samples for IgM dengue by ELISA came positive in one case. This probably was due to the fact that the lumber puncture was done within 24 h of admission and within 7 days of onset of fever in all the five patients of dengue encephalitis and one patient of dengue meningitis.
Conclusion
Neurological manifestations during dengue infection are not uncommon, and it can present in a number of diversified ways. The reemergence of dengue as an important pathogen justifies its inclusion in the differential diagnosis of patients with acute onset of encephalitis and/or encephalopathy in endemic countries or with a travel history suggestive of possible dengue virus exposure. Further epidemiological and neuropathological studies are needed to ascertain the incidence and burden of neurological complications of dengue after a revision of WHO dengue guideline, including case definition for dengue encephalitis and encephalopathy.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- World Health Organization. Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. (2nd ed). Geneva: World Health Organization; 1998.
- [Google Scholar]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. (New edition). Geneva, Switzerland: World Health Organisation; 2009.
- [Google Scholar]
- Dengue infection in patients presenting with neurological manifestations in a dengue endemic population. West Indian Med J. 2008;57:373-6.
- [Google Scholar]
- Neurological complications of dengue fever: Experience from a tertiary center of North India. Ann Indian Acad Neurol. 2011;14:272-8.
- [Google Scholar]
- Encephalitis and myelitis associated with dengue viral infection clinical and neuroimaging features. Clin Neurol Neurosurg. 2008;110:635-40.
- [Google Scholar]
- Dengue encephalitis: Why we need to identify this entity in a dengue-prone region. Singapore Med J. 2006;47:975-7.
- [Google Scholar]
- Neurological complications of dengue virus infection. Lancet Neurol. 2013;12:906-19.
- [Google Scholar]
- Isolation of dengue 2 virus from a patient with central nervous system involvement (transverse myelitis) Rev Soc Bras Med Trop. 2002;35:401-4.
- [Google Scholar]
- Acute transverse myelitis following dengue virus infection. J Clin Virol. 2006;35:310-2.
- [Google Scholar]
- Acute disseminated encephalomyelitis following dengue fever. J Infect Chemother. 2002;8:175-7.
- [Google Scholar]
- Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65:848-51.
- [Google Scholar]
- Dengue infection with central nervous system manifestations. Southeast Asian J Trop Med Public Health. 1999;30:504-6.
- [Google Scholar]
- Breakdown of the blood-brain barrier during dengue virus infection of mice. J Gen Virol. 1991;72:859-66.
- [Google Scholar]
- Neurological complications in dengue infection: A review for clinical practice. Arq Neuropsiquiatr. 2013;71:667-71.
- [Google Scholar]
- Radiological and neurophysiological changes in Japanese encephalitis. J Neurol Neurosurg Psychiatry. 1994;57:1484-7.
- [Google Scholar]
- Thalamus lesions in chronic and acute seizure disorders. Neuroradiology. 2011;53:245-54.
- [Google Scholar]
- Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480-95.
- [Google Scholar]
- Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain. 2003;126:2447-54.
- [Google Scholar]






