Translate this page into:
Changing Pattern of Childhood Epidemic Cerebrospinal Meningitis in North-Western Nigeria
Address for correspondence: Dr. Ibrahim Aliyu, Department of Paediatrics, Aminu Kano Teaching Hospital, Kano, Nigeria. E-mail: ibrahimaliyu2006@yahoo.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Nigeria lies within the meningitis belt which extends from the Gambia, Senegal through Nigeria to Eritrea; however, outbreaks have been shown to extend further south involving countries such as Angola and Namibia. Epidemic outbreaks are often recorded every 8–12 years averaging in a 10 yearly circle however endemic cases still occurs.
Materials and Methods:
The study was retrospective; all results of cerebrospinal fluid (CSF) samples of children with cases of meningitis from January 2010 to December 2010 were collected from the register of the microbiology laboratory of General Hospital Gusau. Relevant information such as their age, sex, CSF macroscopy/microscopy reports, latex particle agglutination test report, and CSF culture report were retrieved and entered into a pro forma.
Results:
There were 89 (73%) males and 33 (27%) females with male to female ratio of 2.7:1. The age ranged from 2 months to 14 years; the mean was 6.27 ± 4.00 years. Meningitis was mostly recorded from January to April. W135 was the most common serotype identified. Majority of the samples (54) which were nonreactive for any of the tested antigens had clear CSF (36), while among those that reacted; the W135 group had a high proportion of cases that had turbid CSF (44); (Fisher's exact test = 30.650, P = 0.000). Majority of the samples (99) had no cell count; although those of the W135 group had higher cell counts followed by those in the nonreactive group (Fisher's exact test = 11.226, P = 0.181).
Conclusion:
Meningitis was highest between January and April, and W135 was the most common serotype.
Keywords
Cerebrospinal fluid culture
epidemic meningitis
latex agglutination
meningitis belt
serotypes
INTRODUCTION
Nigeria lies within the meningitis belt which extends from the Gambia, Senegal through Nigeria to Eritrea; however, outbreaks have been shown to extend further south involving countries such as Angola and Namibia.[12] Epidemic outbreaks are often recorded every 8–12 years averaging in a 10 yearly circle;[3] however, endemic cases still occurs.[456] Neisseria meningitidis is the commonest organism implicated in epidemic meningitis, and 9 serotypes have been identified; these are, namely, A, B, C, D, X, Y, Z, W-135, and 29 E; however, group A and occasionally group C are implicated in most epidemics in sub-Saharan Africa;[7] in an earlier report by Mado et al.[8] 77 cases of cerebrospinal meningitis (CSM) were documented over a 12-month period (2009) in their tertiary institution; however, their result showed that N. meningitidis serogroup A was positive in all of the 77 cerebrospinal fluid (CSF) sampled through latex particle agglutination test (LPA); however, only five positive CSF cultures for N. meningitidis were obtained and an additional five patients had nonmeningococcal meningitis during the epidemics (three pneumococcus and two Haemophilus influenzae). The serotype A had been documented as the predominant strain in most reports of outbreaks in northern Nigeria and Niger Republic.[9] However, this differed from that of Osuorah et al.[10] who reported more of W135 in an outbreak in the Gambia in 2012. However, in 2010, large pool of samples was received from several health facilities in Zamfara state and beyond with diagnosis of meningitis; this study, therefore, seeks to determine the organisms implicated, the serotypes involved and if there was any change in the serotype from those reported in the previous year. Identifying the responsible serotype has important disease preventive role in implementing effective surveillance and vaccination programs.
MATERIALS AND METHODS
The study was retrospective; all results of CSF samples of children with meningitis collected from January 2010 to December 2010 were collected from the register of the microbiology laboratory of General Hospital Gusau. The relevant information such as their age, sex, CSF macroscopy/microscopy reports, LPA test report, and CSF culture report were retrieved and entered into a pro forma. Approval for this study was obtained from the State Ethical Committee on clinical research. The Farida General Hospital Gusau laboratory was the reference center for CSF analysis for the state and its being supported by Federal Ministry of Health, State Ministry of Health and World Health Organization (WHO), so all samples are sent from all health facility and standard WHO protocol for case definition and laboratory procedures are highly maintained.
Materials needed for sample collection
Common materials used were: Trans isolate transport medium (Ti), CSF sampling Kit (L/P kits), and refrigerator for keeping Ti bottles before inoculation.
Sample collection procedure
Before sample collection, the main laboratory was routinely notified; samples were collected by the managing medical officers at referring centers through aseptic technique using the provided LP kit, and 3 ml of CSF were added into sterile CSF containers. Samples collected were transported either by laboratory staffs to the main laboratory; the average time to the main laboratory from the most distant health center was about 40 min. However, considering the fact that the most common etiologic agents of CSM from a previous study[8] are fastidious, 0.5 ml of CSF samples were inoculated onto Trans Isolate Media and the blood onto Brain Heart Infusion Broth immediately after collection when delay in transportation was envisaged.
Other laboratory assays conducted
Pastorex latex agglutination test
Rapid diagnosis (for Streptococcus pneumonia and characterization of specific serogroup for N. meningitidis) based on antigen-antibody test. An equal volume of CSF and antibody was placed on the mat, this is mixed rocked for 15 min, and the presence of agglutination indicates positivity for the specific organism.
Gram and India ink staining
CSF was centrifuged (1500 × g) for 15 min and Gram stain was performed on the centrifuged deposit. One drop of India ink was mixed with one drop of CSF sediment and this was examined under a light microscope, using low (×10) and high dry (×40) magnification for fungal studies. Gram stain and India ink results were reported within 2 h to clinicians.
Cerebrospinal fluid culture procedure
The basic materials for the CSF culture were Loops, Bacti-cinerator, incubator and Co2 jar; while the media used were blood agar (5% sheep or horse blood), MacConkey plates or Chocolate agar with Staphylococcus aureus streak. Standard procedures were followed and in-house quality control measures were ensured. The CSF sediments were inoculated onto two blood agar plates, and streak of S. aureus was added on one of the blood agar to promote the growth of H. influenzae; these were incubated at 35°C–37°C. Blood agar plate was incubated under aerobic and anaerobic condition at 37°C. All media were incubated for 2 days, with daily inspection. However, if after 24 h and there was no growth, we re-incubated for an additional 24 h.
Reporting reference limits
-
Preliminary reports after 24 h were sent to the attending physician: “No growth after 24 h’ incubation” or “growth after 24 h’ incubation. Antibiotic susceptibility results to follow”
-
The final results were issued when definitive organism identification and antibiotic test results were available.
Statistical analysis
Obtained data was cleaned and entered into Statistical Package for Social Sciences, (SPSS) version 20; (IBM SPSS Corp Armonk, New York, United State of America). The data were presented as tables, graphs, and frequencies. Test of significance using the Fisher's exact test was deployed with P < 0.05 been set as statistically significant.
RESULTS
There were 89 (73%) males and 33 (27%) females with male to female ratio of 2.7:1. The age range of the individuals was from 2 months to 14 years; the mean age was 6.27 ± 4.00 years.
Samples for cases of meningitis were mostly recorded from January to April and the highest record was March (89; 73%) while April (1; 0.8%) had the least; February and January had 28 (23%) and 4 (3.3%), respectively [Figure 1].
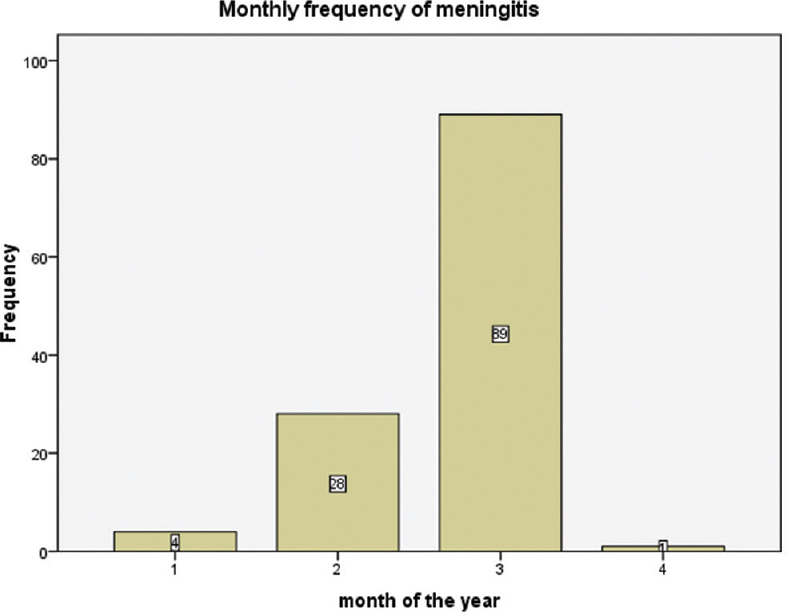
- Monthly distribution of cases of meningitis
Majority of the samples were received from subjects who were within their childhood (36.1%) while infants were the least (13.1%). Most of the samples were from participants within the 1–5 years of age group (41.8%) while those <1 year were the least (6.6%) [Table 1].

Most of the samples were turbid at submission (74; 60.7%) while 48 (39.3%) were clear. The mean cell count was 0.8 × 109/L ± 1.9 × 109/L; absent cell (81.1%) in the CSF was the most common observation, while only 9 (7.4%) samples had more than 5 cells in the CSF [Table 2].

N. meningitidis serotype W135 was the most common serotype identified, while 44.3% of them were nonreactive, serotype A was the least recorded.
Majority of the samples (54) which were nonreactive for any of the tested antigen had a clear CSF (36), while among those that were reactive; the W135 group had high proportion of cases that had turbid CSF (44); this observation was noted to be statistically significant (‡Fisher's exact test = 30.650, P = 0.000).
Higher proportion (62) of the samples with absent cells appeared turbid at submission, however, all the samples with the highest cell counts were turbid at presentation, this observation was not statistically significant (†Fisher's exact test = 6.006, P = 0.091) [Table 3].

Majority of the samples (99) had no cell count (0) although those of the W135 group had higher cell counts followed by those in the nonreactive group, however it was not statistically significant (Fisher's exact test = 11.226, P = 0.181) [Table 4].

DISCUSSION
Meningococcal disease outbreaks occur in the semi-arid region of sub-Saharan Africa which has been described by the WHO as the meningitis belt; northern Nigeria lies within this description, therefore, reports of epidemics are not uncommon; however, infection caused by other strains of bacteria and nonbacterial organisms are also observed in the region. Outbreaks of CSM often occur during the hot season between December to June.[11]
This study showed that more males were affected than females, this observation was similar to that reported by Tavasoli et al.[12] and Heydarian et al.[13] both in Iran; similarly, Lagunju et al.[14] in Ibadan and Frank-Briggs and Alikor[15] in Port-Harcourt reported the same pattern of male predominance; why this is so is not clearly understood, however, the role of sex variation in the development of the immune system and susceptibility to various bacterial infection as was reported by Green[16] is plausible.
Samples were mostly received from January to April and March had the highest; this observation was slightly different from the previous reports;[1718] Mado et al.[8] in the 2009 CSM outbreak, cases were recorded up to May; and April recorded the highest cases followed by March.
The W135 serotype was the predominant strain in this study; this was a great departure from previous reports[89] which showed A serotype as the most common cause of outbreak; however, our result was similar to that of Osuorah et al.[10] in their report from the Gambia. The world is a global village; with the ease of travel introduction of alien serotypes is not an uncommon phenomenon.
The age distribution in this study was similar to the previous reports of Odedina and Emumwen,[19] Mado et al.;[8] (those <1 year constituting 6.6%) and Emele and Anyiwo[20] but contrasted with that of Mohammed et al.[21] and Bwala et al.[22] who reported high incidence in infancy. They attributed their experience to the impact of virulence of the subgroups involved in their outbreak.
Seventy-four (60.7%) of the sample were reported turbid; this was higher than the 2% reported by Odedina and Emumwen;[19] the reason for the difference was not clear, however, turbidity is subjective, therefore milder grades of severity are often prone to either been under or overestimated. However, the culture yield was low in this study when compared to 6.5% by Mado et al.[8] and 14% by Odedina and Emumwen;[19] no positive culture was obtained in this study.
Ninety-nine (81.1%) of the samples had absent neutrophil in the CSF; absent CSF neutrophilic pleocytosis has been associated with meningococcal meningitis,[23] especially following early lumbar puncture sampling; therefore, repeat sampling is advised in such settings. Furthermore, our laboratory received samples from distant health facilities; therefore, the possibility of degradation was also entertained.
CONCLUSION
This study showed that epidemic meningitis was highest between January and April, and the predominant serotype (W135) was a great departure from the usual; therefore, this highlights the significant of continuous surveillance, which facilitates efficient vaccination program.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Where is the meningitis belt.Defining an area at risk of epidemic meningitis in Africa? Trans R Soc Trop Med Hyg. 2002;96:242-9.
- [Google Scholar]
- Meningococcal meningitis: Etiology, diagnosis, epidemiology and treatment. Am J Med Med Sci. 2014;4:266-71.
- [Google Scholar]
- Profiles of acute bacterial meningitis isolates in children in national hospital, Abuja. Niger Med J. 2015;56:297-300.
- [Google Scholar]
- Epidemiology and prognosis of pyogenic meningitis in Nigerians – A study of 294 patients. Niger Med J. 1976;6:152-9.
- [Google Scholar]
- Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196-210.
- [Google Scholar]
- Epidemic cerebrospinal meningitis in children at Federal Medical Centre, Gusau, Zamfara state, Nigeria. Niger J Paediatr. 2013;40:169-71.
- [Google Scholar]
- World Health Organization. Control of Epidemic Meningococcal Diseases. WHO Practical Guidelines. Geneva, WHO: Lyons Foundation, Merieux; 1995.
- Outbreak of serotype W135 Neisseria meningitidis in central river region of the Gambia between February and June 2012: A hospital-based review of paediatric cases. Niger J Clin Pract. 2015;18:41-7.
- [Google Scholar]
- World Health Organization. Meningitis Outbreak in Nigeria and Niger. Available from: http://www.who.int/csr/don/13-march-2015-nigeria/en/
- Frequency of meningitis in children presenting with febrile seizures at Ali-Asghar children's hospital. Iran J Child Neurol. 2014;8:51-6.
- [Google Scholar]
- Predicting factors and prevalence of meningitis in patients with first seizure and fever aged 6 to 18 months. Neurosciences (Riyadh). 2014;19:297-300.
- [Google Scholar]
- Childhood bacterial meningitis in Ibadan, Nigeria – Antibiotic sensitivity pattern of pathogens, prognostic indices and outcome. Afr J Med Med Sci. 2008;37:185-91.
- [Google Scholar]
- Long term neurological complications of bacterial meningitis in Nigerian children. Niger J Paediatr. 2013;40:295-8.
- [Google Scholar]
- The male predominance in the incidence of infectious diseases in children: A postulated explanation for disparities in the literature. Int J Epidemiol. 1992;21:381-6.
- [Google Scholar]
- Clinical features of epidemic meningococcal meningitis. Niger Med J. 1977;4:369-73.
- [Google Scholar]
- Meningococcal meningitis in the northern savanna of Africa. Trop Doct. 1976;6:99-104.
- [Google Scholar]
- Bacterial meningitis among children in Federal Medical Centre. Afr J Clin Exp Microbiol. 2008;9:152-6.
- [Google Scholar]
- Cerebrospinal meningitis in Sokoto Nigeria: A bacteriological analysis of three successive epidemics. J Med Lab Sci. 1994;4:34-9.
- [Google Scholar]
- A severe epidemic of meningococcal meningitis in Nigeria, 1996. Trans R Soc Trop Med Hyg. 2000;94:265-70.
- [Google Scholar]
- An outbreak of meningococcal meningitis in Maiduguri, Borno State, Nigeria: I.Cinical aspects. Niger Med Pract. 1989;18:31-3.
- [Google Scholar]
- Bacterial meningitis in the absence of cerebrospinal fluid pleocytosis: A case report and review of the literature. Can J Infect Dis Med Microbiol. 2014;25:249-51.
- [Google Scholar]






