Translate this page into:
Cavernous Carotid Aneurysms: To Do or Not To Do?
Address for correspondence: Dr. R. Girish Menon, Department of Neurosurgery, Kasturba Medical College, Manipal - 576 104, Karnataka, India. E-mail: girish.menon@manipal.edu
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cavernous carotid aneurysms (CCA) pose considerable dilemmas in management. It is still unclear as to whether an asymptomatic CCA should be subjected to treatment. Similarly, the ideal management strategy for a symptomatic aneurysm is controversial. We present the case of a 60-year-old female with a giant CCA and discuss the management issues.
Keywords
Cavernous carotid aneurysms
cerebral bypass
unruptured aneurysms
INTRODUCTION
Cavernous carotid aneurysms (CCA) pose considerable dilemmas in management. It is still unclear as to whether an asymptomatic cavernous carotid aneurysm should be subjected to treatment. Similarly, the ideal management strategy for a symptomatic aneurysm is controversial. We present the case of a 60 years old lady with a giant CCA and discuss the management issues.
CASE REPORT
A 60-year-old hypertensive female presented with complaints of blurring of vision and left hemifacial and periorbital pain of 2 months duration. One month into her illness, she developed drooping of her left eyelid. On admission, the general systemic examination was normal. She had diminished visual acuity of her left eye and could only perceive hand movements. She had complete ophthalmoplegia of her left eye along with sensory blunting involving the first two divisions of the left trigeminal nerve. Rest of her nervous system examination was normal. Her magnetic resonance imaging [Figure 1] revealed a well-defined extra-axial altered signal intensity lesion measuring 4.0 cm × 4.1 cm × 3.4 cm in the left parasellar region which was iso to hyperintense on T1-weighted imaging, heterogeneous on T2-weighted/fluid-attenuated inversion recovery and did not show any diffusion restriction. On postcontrast studies, the central portion of the lesion showed homogeneous intense enhancement while rest of the lesion did not show enhancement. The left optic nerve appeared displaced and pushed medially and upward. The sphenoid bone was unremarkable. Digital subtraction angiography [Figure 2] revealed a giant cavernous aneurysms measuring nearly 3.5 cm × 2.4 cm with areas of thrombosis. An aneurysm was seen extending superiorly into the intradural subarachnoid compartment. Cross circulation studies following compression of the left carotid artery revealed inadequate filling of the ipsilateral middle cerebral vessels, but there was a venous filling delay of over 5 s in the ipsilateral side. She underwent a high flow extracranial-intracranial (EC-IC) (proximal internal carotid artery [ICA] to M2 segment of middle cerebral artery) bypass using a saphenous vein graft followed by ICA ligation. Postoperative computed tomography angiogram [Figure 3] revealed complete thrombosis of the aneurysm with no evidence of contrast enhancement. The bypass graft showed normal contrast opacification and there was no flow detected in the ICA distal to the occlusion.

- Magnetic resonance imaging (MRI) revealed a well defined extra axial altered signal intensity lesion measuring 4.0 x 4.1 x 3.4 cm in the left parasellar region which was heterogenous on T2W/FLAIR (a and c) iso to hyperintense on T1WI (b), and did not show any diffusion restriction. On post contrast (d) studies the central portion of the lesion showed homogenous intense enhancement while rest of the lesion did not show enhancement
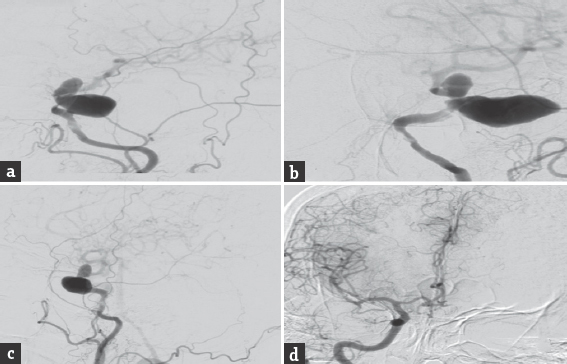
- Digital subtraction angiography anteroposterior, lateral and oblique images (a-c) revealed a giant cavernous aneurysms measuring nearly 3.5 cm × 2.4 cm with areas of thrombosis. The aneurysm was seen extending superiorly into the intradural subarachnoid compartment. Cross circulation studies following compression of the left carotid artery revealed inadequate filling of the ipsilateral middle cerebral vessels (d)
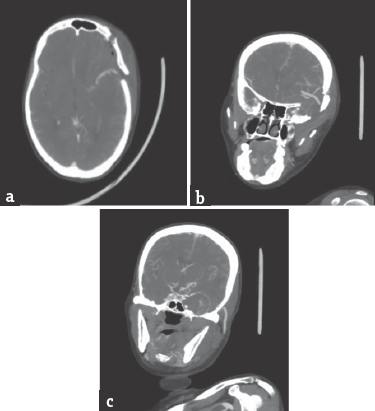
- Postoperative computed tomography angiogram revealed complete thrombosis of the aneurysm with no evidence of contrast enhancement. (c) The bypass graft showed normal contrast opacification (a and b) and there was no flow detected in the internal carotid artery distal to the occlusion (c)
DISCUSSION
Cavernous carotid aneurysms (CCAs) constitute 2%–9% of all IC aneurysm.[1] These aneurysms are can be idiopathic, traumatic, iatrogenic, or infectious in etiology. Traumatic, iatrogenic, and infectious aneurysms have an aggressive course and need urgent intervention.[2] The natural history of idiopathic cavernous aneurysms is not well known. They often remain asymptomatic and are detected incidentally. They tend to become large to giant in size when they manifest with features of mass effect in the form of cranial nerve palsies of adjacent nerves. This could be in the form of diplopia, ptosis, ophthalmoplegia, or pain or paresthesia along the fifth nerve distribution. A Large transitional variant of these aneurysms which have an intradural component can press against the optic nerve and result in visual symptoms. These intradural variants also carry a risk of subarachnoid hemorrhage (0.2%–0.4%).[34] Intracavernous rupture can result in direct caroticocavernous fistula although such instances are rare.[2] Rarely, these aneurysms can erode into the sphenoid sinus and rupture resulting in fatal epistaxis. Spontaneous thrombosis of these aneurysms has also been reported as have been thromboembolic strokes originating from intra-aneurysmal thrombus.[56]
The dilemmas in the management essentially pertain to (a). What are the chances that an asymptomatic CCA would turn symptomatic and is prophylactic treatment indicated in such aneurysms? (b) What are the treatment options for a symptomatic CCA?
Unruptured asymptomatic aneurysms
These aneurysms are known to have an overall low risk of rupture and life-altering complications. Being enclosed in safe venous pouch, the cavernous sinus, these aneurysms tend to grow from large to giant size before they manifest clinically. In the ISUIA study, the 5-year cumulative rupture rate of unruptured CCAs was 0% for aneurysms with size ≤12 mm, 3% for aneurysms of size 13–24 mm and 6.4% for aneurysms >5 mm.[7] Similarly, risk prediction for bleed based on the PHASES score remains high for these aneurysms as the majority of these aneurysms tend to be giant.[8] However, for some reason, the common predictors of bleed for an incidental aneurysm such as hypertension, age, sex, and previous bleeds, do not apply strictly for CCAs There are several published series on successful conservative management of cavernous segment aneurysms the largest being by Stiebel-Kalish et al.[9] In their series of 132 aneurysms followed up for 4 years, 39 patients improved 21 remained unchanged, eight patients worsened and two died, suggesting a rather benign course compared to other aneurysms. The need for any prophylactic intervention purely based on the size of the aneurysm, thus, remains debatable.
Intervention for an asymptomatic aneurysm is justified in three situations. Aneurysms with an intradural component carry a risk of subarachnoid hemorrhage and need to be promptly excluded from the circulation. Such intradural components are often missed if not looked for specifically. Appropriate imaging of the dural rings is, therefore, mandatory for all CCAs. Similarly, an expanding aneurysm can result in sphenoid bone erosion, which can eventually result in fatal epistaxis. The presence of sphenoid bone erosion on bone window scans is the second indication for prophylactic intervention in an asymptomatic aneurysm. The third instance for preemptive treatment for a CCA would be an aneurysm seen to have increased in size on serial angiograms, especially in a young individual.
Symptomatic aneurysms
It is reasonable to assume that all symptomatic CCAs should be treated. Symptomatic aneurysms which have bled, which produce thromboembolism, which cause intolerable pain and deteriorating vision definitely merit treatment. Cranial nerve palsies other than vision, association with previously ruptured aneurysms, etc., are gray areas where treatment needs to be individualized. However, treatment is justified only if a successful cure can be offered without major complications. Both surgical and endovascular modalities of treatment essentially attempt to exclude the aneurysm from the circulation and initiate thrombosis. Except for direct clipping of the aneurysm none of the other modalities attempt to decompress the aneurysm and thereby reduce mass effect. Pain is one symptom which responds moderately well to treatment. Successful intervention certainly reduces the risk of further bleed and ischemic stroke. Intervention helps in arresting the progression of cranial nerve deficits and complete recovery seldom happens. The second cranial nerve is an exception and visual recovery following decompression has been reported in few cases.[12] Like other causes of cranial nerve deficits, the duration of the deficits before treatment can have an impact on the resolution of the deficit.
Unfortunately, all modalities carry a considerable risk of complications. Both endovascular and surgical options carry significant risks, and the overall mortality and morbidity varies from 3.23%–22.6% to 9.2%–14.8%, respectively.[1] The decision to intervene thus needs to be made judiciously and the type of treatment ought to be justified adequately.
Treatment modalities
Exclusion of the aneurysm from the circulation can be done either by surgery or by endovascular techniques. Treatment can be either occlusive or reconstructive. Occlusive strategies include parent artery ligation surgically or by endovascular techniques. Reconstructive strategies include direct microsurgical clip application, coil embolization with or without the use of a vascular reconstruction device, flow-diverting devices, or the use of liquid embolic agents. With the advent of flow diverters, the results have improved with an obliteration rate of 60%–80% with morbidity ranging from 10.44% (9.9%–15.2%) and mortality of 6.86% (2.3%–9.2%).[1] However, in developing countries like India, cost is a major limitation for endovascular techniques, and surgical option remains the mainstay of treatment.
Surgery
Literature provides reports of <150 cases of CCA which have undergone direct clipping so far. Direct clipping probably provides the chance of near 100% obliteration maintaining parent vessel patency at the same time. Of these Dolenc[1011] with 115 cases have the largest series and report a morbidity of 9.5% and morbidity 2.6% while Diaz et al.[12] with 15 cases report a 20% morbidity and 6.6% risk of mortality. However, the technique is complex and carries a high complication rate. Other authors have not been able to reproduce the same results and report a high risk of morbidity (17.45%) and mortality (31.27%).[1] Direct clipping is thus seldom preferred.
Occlusive surgical options include proximal carotid ligation with or without trapping the aneurysmal carotid segment by placing a clip proximal to the ophthalmic artery. Proximal carotid ligation carries a risk of ischemia to the ipsilateral hemisphere. It also carries a risk of new aneurysm formation on the contralateral side or increase in the size of any contralateral aneurysm if any. Risk of ischemia can be predicted by various techniques such as rate of venous filling, balloon test occlusion (BTO), single-photon emission computed tomography (SPECT), and positron emission tomography (PET). The risk of infarct is 32%–60% if carotid ligation is carried out without any preoperative assessment of cardiovascular reserve.[13141516] Tests of cerebrovascular reserve like BTO, SPECT, etc., are at times difficult to perform. A simple alternative is to look for a delay of more than 0.5 s in venous filling in the vascular territory of the occluded vessel which indicates nontolerance. This simple test described by Müller-Forell and Valavanis in 1995 and later validated by van Rooij et al. is a simple safe and effective in predicting tolerance to carotid occlusion and has become the current standard of practice in many centers.[1718] The risk of infarction comes down to 22% in patients who successfully complete all preoperative assessment tests.[1920] The risk, however, does not completely disappear. With an additional bypass, this risk comes down to 14.6% (1.8–29.4). A bypass procedure before parent artery occlusion is, therefore, preferable to reduce the risks of postocclusion stroke even in patients who tolerate BTO successfully (universal bypass).
Bypass could be an augmentative Superficial temporal to Middle cerebral artery (low flow STMC) bypass for patients with moderate cerebrovascular reserve or a replacement bypass (high flow EC-IC) bypass for those with poor cerebrovascular reserve. The choice between an arterial radial artery graft and a venous saphenous graft depends on the surgeon's preference. Radial artery graft lasts longer but has potential risk of spasm. Venous graft is easier to harvest but is prone to kinking and occlusion. Similarly, the choice between parent artery occlusion alone or trapping is difficult as it has been shown that there is no difference in complications and outcome between trapping and carotid occlusion.[13] Trapping supposedly prevents backflow into the aneurysm due to cross circulation and also prevents dislodged thrombi from reaching the IC circulation. Trapping is preferred to mere carotid occlusion for aneurysms with significant intradural extension and patients who demonstrate significant retrograde flow during the BTO.[21] The next controversy pertains to the choice of the proximal site for anastomosis. Common carotid artery, ICA and external carotid artery all have comparable results and the decision essentially depends on the surgeon's preference. We prefer the ICA or the external carotid artery to avoid manipulation of the carotid bulb situated close to the bifurcation.
Our patient presented with poor vision and intolerable pain justifying treatment. We did not perform a BTO, SPECT, or PET study for our patient as the cross compression study revealed poor cross circulation. Saphenous vein graft and ICA for proximal anastomosis was chosen due to surgeon's preference and past experience.
CONCLUSION
CCA are rare and pose considerable challenges in management. Their natural history is unclear, but they seldom cause life-threatening complications. The decision to treat needs to be made judiciously based on careful interpretation of the radiological images. Venous filling on cross compression is a good indicator for cross circulation and obviates the need for detailed cerebrovascular reserve studies in selected cases. Endovascular treatment strategies offer reasonable results but are expensive. Parent artery occlusion combined with a universal bypass is a cost-effective alternate option with comparable results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Evolution of management strategies for cavernous carotid aneurysms: A review. World Neurosurg. 2014;82:1077-85.
- [Google Scholar]
- Cavernous carotid aneurysms: You can but should you? World Neurosurg. 2014;82:996-7.
- [Google Scholar]
- Carotid ligation for intracranial aneurysm; a follow-up study of 54 patients. J Neurosurg. 1958;15:281-9.
- [Google Scholar]
- Spontaneous thrombosis of giant cavernous internal carotid artery aneurysm in a neonate. Case report and review of the literature. Pediatr Neurosurg. 2008;44:329-32.
- [Google Scholar]
- Giant mycotic aneurysm of the internal carotid artery in a child: Endovascular treatment. Pediatr Radiol. 2003;33:211-5.
- [Google Scholar]
- Carotid cavernous fistula: Direct repair with preservation of the carotid artery. Technical note. J Neurosurg. 1973;38:99-106.
- [Google Scholar]
- International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured tracranial aneurysms – Risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-33.
- [Google Scholar]
- Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59-66.
- [Google Scholar]
- Presentation, natural history, and management of carotid cavernous aneurysms. Neurosurgery. 2005;57:850-7.
- [Google Scholar]
- Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983;58:824-31.
- [Google Scholar]
- Extradural approach to intracavernous ICA aneurysms. Acta Neurochir Suppl. 1999;72:99-106.
- [Google Scholar]
- Surgical management of aneurysms in the cavernous sinus. Acta Neurochir (Wien). 1988;91:25-8.
- [Google Scholar]
- Results following ligation of the internal carotid artery. Arch Surg. 1942;45:521-33.
- [Google Scholar]
- A new method to predict safe resection of the internal carotid artery. Laryngoscope. 1990;100:85-8.
- [Google Scholar]
- Aneurysms of the intracavernous carotid artery: Clinical presentation, radiographic features, and pathogenesis. Neurosurgery. 1990;26:71-9.
- [Google Scholar]
- Aneurysms of the intracavernous carotid artery: A multidisciplinary approach to treatment. J Neurosurg. 1991;75:525-34.
- [Google Scholar]
- Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. 2005;26:175-8.
- [Google Scholar]
- How angioarchitecture of cerebral arteriovenous malformations should influence the therapeutic considerations. Minim Invasive Neurosurg. 1995;38:32-40.
- [Google Scholar]
- Unruptured aneurysms of the intracavernous internal carotid artery: Outcome following carotid ligation or conservative treatment. Br J Neurosurg. 1989;3:181-8.
- [Google Scholar]
- Results of treatment of intracranial aneurysms using the Selverstone clamp. J Neurosurg. 1959;16:611-8.
- [Google Scholar]
- Treatment of intracavernous and giant carotid aneurysms by combined internal carotid ligation and extra- to intracranial bypass. J Neurosurg. 1980;52:1-10.
- [Google Scholar]






