Translate this page into:
”Bovine Aortic Arch” in a Patient with Simultaneous Bihemispheric Embolic Infarcts
Address for correspondence: Dr. Prasad Krishnan, Department of Neurosurgery, National Neurosciences Centre, Peerless Hospital Campus, 2nd Floor, 360 Panchasayar, Kolkata - 700 094, West Bengal, India. E-mail: prasad.krishnan@rediffmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 60-year-old male who had suffered a myocardial infarction 3 weeks previously presented with sudden-onset aphasia and weakness of all four limbs. Computed tomography (CT) scan showed bilateral frontoparietal hypodensities [Figure 1]. Magnetic resonance imaging showed [Figure 2] bilateral infarcts (of the same age) with patchy hemorrhagic transformation. Due to the discrete bihemispheric location of the infarcts, an embolic cause was suspected. To delineate the cause, initially, an echocardiogram was done that showed akinetic and thin inferoposterior wall with dilated left atrium and mild mitral regurgitation with impaired left ventricular systolic function (ejection fraction 46%). A Holter study showed no evidence of atrial fibrillation. Then, a CT angiogram of the great vessels was done that showed an anomalous origin of the left common carotid artery (CCA) from the innominate artery (IA) [Figure 3]. Finally, a transesophageal echocardiography was done that showed the presence of a clot in the left atrium.
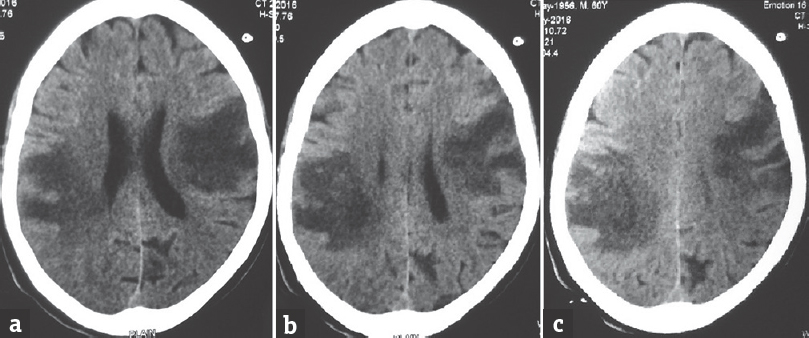
- Axial noncontrast computed tomography scan sections showing bihemispheric hypodensities in frontoparietal regions
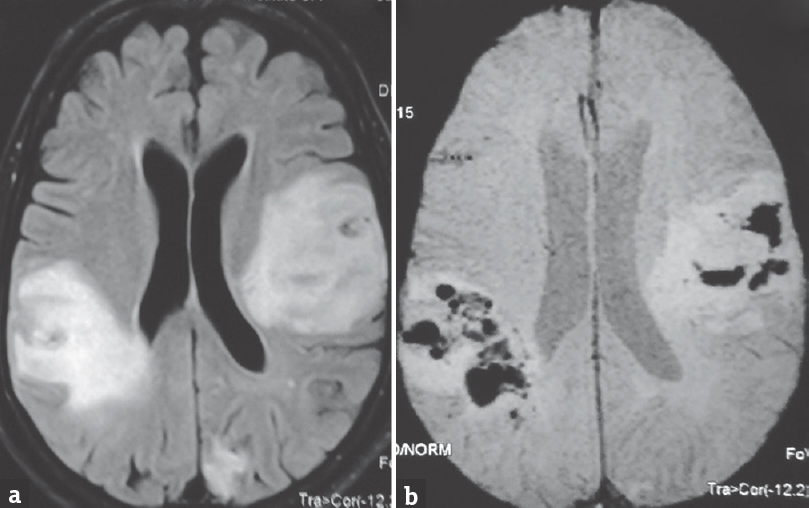
- Axial magnetic resonance imaging showing bilateral hyperintensities suggestive of infarct in (a) fluid attenuation inversion recovery sequences with evidence of hemorrhagic transformation in (b) gradient echocardiography imaging sequences
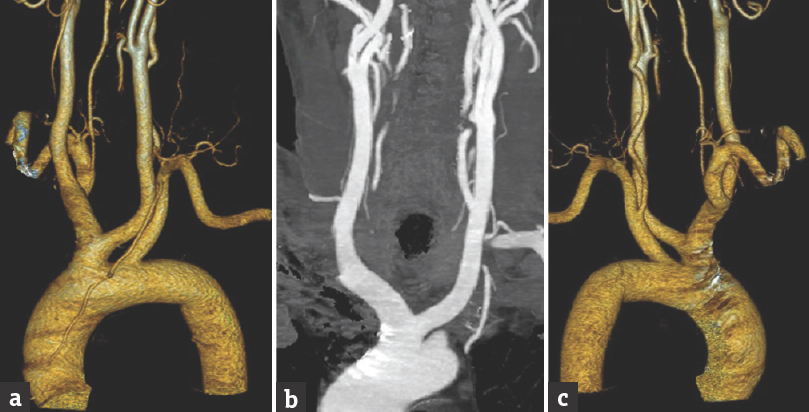
- Computed tomography angiography images with three-dimensional reconstruction (a) anterior view and (c) posterior view showing common origin of the left common carotid artery and innominate artery from the aortic arch (b) coronal computed tomography image with contrast showing both common carotid artery resembling horns of a bull
In humans, the commonly encountered branching pattern from the arch of the aorta consists of the IA, the left CCA and the left subclavian artery originating separately.[1] The variation seen in our patient consisted of the left CCA having a common origin with the IA from a single vascular trunk. This variant is most often termed a “bovine aortic arch.”[1] Others describe a more distal origin of the left CCA from the IA itself as the “bovine arch.”[123] However, the term “bovine aortic arch” in both these instances is a misnomer whose origin is unknown[2] since in cattle, a single great vessel originates from the aortic arch and trifurcates into both subclavian arteries and a bicarotid trunk (which subsequently divides to the right and left CCAs, respectively).[1] Vitek[4] has commented on this “discordance of terminology” and suggested that the radiological resemblance to the 2 horns on the head of the cow might have led to this term.
This variant occurs due to slower growth of the 3rd and 4th ventral aortic roots in fetal life resulting in the common origin of the left CCA and IA.[3] We hypothesize that in our patient, the relatively large size of the combined IA and left CCA origin led to a “streaming phenomenon”[3] that might have sucked in a dislodged cardiac thrombus and caused simultaneous embolic infarcts in both the cerebral hemispheres.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Bovine aortic arch variant in humans: Clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27:1541-2.
- [Google Scholar]
- Bovine aortic arch: A novel association with thoracic aortic dilation. Clin Radiol. 2012;67:28-31.
- [Google Scholar]





