Translate this page into:
Association of Serum Calcium Levels with Infarct Size in Acute Ischemic Stroke: Observations from Northeast India
Address for correspondence: Dr. Alice Abraham Ruram, Department of Biochemistry, North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong, Meghalaya, India. E-mail: ruramalice9@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Calcium is known to be major mediator in ischemic neuronal cell death. Recent studies have shown that elevated serum calcium levels at admission in patients with stroke have been associated with less severe clinical deficits and with better outcomes.
Aim:
The aim of this to determine the correlation between serum calcium (total, corrected, and ionized) and infarct size (IS) in patients with acute ischemic stroke.
Materials and Methods:
Data were collected from 61 patients presenting with acute ischemic stroke from May 2015 to April 2016 at a tertiary care institute in Northeast India. Only patients aged ≥40 years and diagnosed as having acute ischemic cerebrovascular stroke with clinical examination and confirmed by a computed tomography scan were included in the study. Serum calcium levels (total, albumin corrected, and ionized) were collapsed into quartiles, and these quartile versions were used for calculating correlation. Pearson's correlation coefficient was used for comparing calcium levels with IS.
Results:
Total calcium, albumin-corrected calcium, and ionized calcium had a statistically significant negative correlation with IS with r = −0.578, −0.5396, and −0.5335, respectively. Total and ionized calcium showed a significant negative correlation with IS across all four quartiles. Albumin-corrected calcium levels showed a significant negative correlation with IS only across the lowest and highest quartiles.
Conclusion:
The findings in our study suggest that serum calcium can be used as a prognostic indicator in ischemic stroke as its levels directly correlates with the IS.
Keywords
Acute ischemic stroke
albumin-corrected calcium
infarct size
ionized calcium
total calcium
INTRODUCTION
Stroke is defined by the World Health Organization as “a clinical syndrome typified by rapidly developing signs of focal or global disturbance of cerebral functions, lasting more than 24 h or leading to death, with no apparent causes other than of vascular origin.”[1] Stroke, along with its devastating and long-term complications are one of the leading causes of morbidity and mortality in the world as well as in India. India, with more than 1 billion inhabitants, is undergoing remarkable economic and demographic changes in the recent years resulting in a transition from poverty-related infectious and nutritional deficiency diseases toward lifestyle-related cardiovascular and cerebrovascular diseases.[23] Considering the immense medical, financial and social burden ischemic stroke confers, the need to develop reliable and viable prognostic markers remains an important goal in the field of medical research.
Normal cerebral blood flow (CBF) in man is typically in the range of 45–50 ml/min/100 g between a mean arterial pressure of 60 and 130 mmHg.[4] When CBF falls below 20–30 ml/min/100 g, marked disturbances in brain metabolism begin to occur, such as water and electrolyte shifts and regional areas of the cerebral cortex experience failed perfusion. At blood flow rates below 10 ml/min/100 g, sudden depolarization of the neurons occurs with depletion of high-energy compounds such as adenosine triphosphate (ATP), rapid loss of intracellular potassium (K+) to the extracellular space, and sodium (Na+) and calcium (Ca2+) begin to enter the cells.[5]
Normally, the 10,000:1 (extracellular: intracellular) differential is maintained by at least four mechanisms, three of which are ATP dependent[678] Ischemia leads to interruption in the oxygen-dependent generation of high-energy compounds eliminating three of the four mechanisms of cellular calcium homeostasis. A rapid and massive influx of calcium into the cell results.[9] The only remaining mechanism: mitochondrial sequestration causes overloading of the mitochondria with calcium and diminished capacity for oxidative phosphorylation. Elevated intracellular Ca2+ activates membrane phospholipases and protein kinases. A consequence of phospholipase activation is the production of free fatty acids (FFA’s) including the potent prostaglandin inducer, arachidonic acid (AA). The degradation of the membrane by phospholipases almost certainly damages membrane integrity, further reducing the efficiency of calcium pumping and leading to further calcium overload, and a failure to regulate intracellular calcium levels following the ischemic episode.[10] In addition, FFAs almost certainly have other degradative effects on cell membranes.[11] The production of AA as a result of FFA release causes a biochemical cascade ending with the production of thromboxane and leukotrienes. Both these compounds are profound tissue irritants which can cause platelet aggregation, clotting, vasospasm, and edema,[111213] with resultant further compromise to the restoration of adequate cerebral perfusion on the restoration of blood flow.
Awareness of the participation of Ca2+ in the ischemic cascade has led to the development of several potential neuroprotective agents designed to modify the role of this ion in acute focal brain injury. However, most endeavors have proven not very successful presumably because of existence of several types of calcium channels which continue to allow calcium entry into the cells, despite blockage of one particular type of calcium channel. However, an underlying role of movement of calcium from the extracellular to the intracellular compartment leading to triggering of the cascade of events that eventually lead to neuronal cell death is undebatable.
A study carried out by Chung et al. found that higher albumin-corrected calcium levels were of prognostic significance in terms of early neurologic outcome and long-term mortality after acute ischemic stroke.[14] Serum calcium also correlates with the size of cerebral infarct and clinical outcomes as shown by Buck et al. and Ovbiagele et al.[1516] Despite the rapid increase in the incidence of stroke in India, as well as the Northeastern part of the country, there is a dearth of such studies from these regions. As such, our study was an endeavor to study the significance of serum calcium levels in stroke, as well to find the correlation, if any of serum calcium (total, ionized and albumin corrected) with infarct size (IS) in ischemic stroke.
MATERIALS AND METHODS
Study design
This study was conducted at a tertiary care institute in Shillong, Meghalaya. Data were collected from all patients admitted with ischemic stroke for 1 year from May 2015 to April 2016. The study protocol was approved by the Institutional Ethics Committee, and the written informed consent was taken from all participants before their inclusion in the study. Only patients aged ≥40 years and diagnosed as having acute ischemic cerebrovascular stroke with clinical examination and confirmed by a computed tomography (CT) scan were included in the study. Patients aged <40 years, presenting with hemorrhagic stroke, subarachnoid hemorrhage, and cerebral venous sinus thrombosis were excluded from the study. Patients with hepatic or renal disease were also excluded as these might affect calcium or albumin levels. Out of 126 patients who had presented with stroke, only 61 patients were included considering all relevant inclusion and exclusion criteria.
Measurement of total (TCa) and ionized (ICa) calcium was done in Caretium ISE analyzer. Albumin-corrected calcium (CCa) level was calculated as serum total calcium level + 0.8 × (4 − patient's albumin level) (Normal serum albumin level is 4 mg/dL). CT imaging was performed with a Multislice (128 slices) CT scan (Definition AS + Excel Seimens, Germany, No-1) in all patients. For analysis of the IS, the formula shown by Sims et al. to be the most accurate was used.[17] As per this formula, the largest lesion slice was selected. The longest lesion axis on this slice was measured with the ruler tool. A second line was drawn perpendicular to the first at the widest dimension. These two measurements were called the x (A) and y (B) axes. A third axis, the z (C) axis, was computed by multiplying the number of slices by slice thickness. The scan slice for CT was 5 mm. Final IS was measured as ABC/2. For the purpose of the study, biochemical and radiological investigations done immediately after admission (<48 h) were used.
Statistical analysis
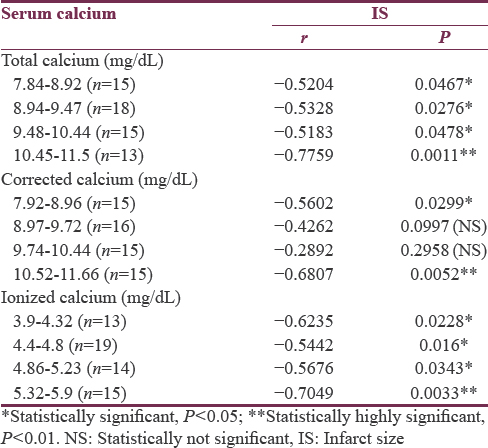
A database was constructed on Microsoft Excel (Office 2007, Microsoft Corporation, Redmond, Washington, USA), and the statistical analyses were performed using GraphPad Prism version 7.00 (GraphPad Software, Inc., California, USA). Continuous variables were shown as mean ± standard deviation, and nominal variables were shown as the number of cases and percentage. Serum calcium levels (total, albumin corrected, and ionized) were collapsed into quartiles (values provided in tables), and these quartile versions were used to calculate correlation. Pearson's correlation coefficient was used for comparing TCa, ICa, and CCa with IS. A two-tailed P < 0.05 was considered statistically significant, whereas a P < 0.01 was considered highly significant.
Results and Observations
Data were collected from 61 patients out of 126 patients who had presented with cerebrovascular accident after due consideration to all relevant inclusion and exclusion criteria. There were 23 females (37.71%) and 38 males (62.29%), ranging from 40 years to 81 years in age. The mean age of the patients was 59.31 ± 11.04 years. The levels of total calcium, albumin-corrected calcium, and ionized calcium were 9.61 ± 0.96 mg/dL (range: 7.84–11.5), 9.72 ± 0.97 mg/dL (range: 7.92–11.66), and 4.82 ± 0.51 mg/dL (range: 3.9–5.9), respectively. Mean IS as measured on the CT scan was 45.44 ± 18.2 cm3 (range: 16–94). The levels of total protein and albumin in the patients with ischemic stroke were 7.43 ± 0.61 g/dL (range: 5–8.4) and 3.87 ± 0.39 g/dL (range: 2.3–4.9), respectively. Gender-wise distribution of baseline biochemical and radiological parameters are shown in [Table 1]. Analysis revealed no significant difference between males and females with respect to age, calcium levels (total, corrected, and ionized), total protein, albumin levels, or IS.
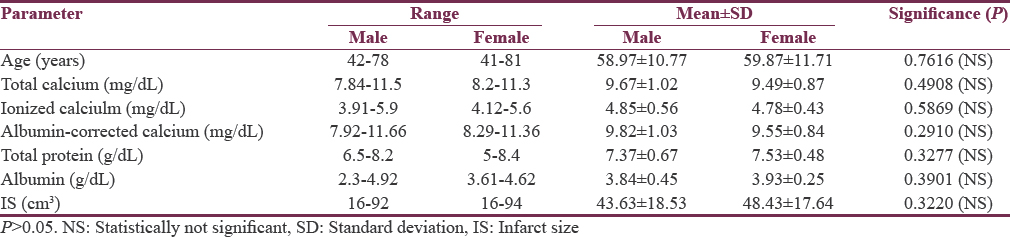
Total calcium, albumin-corrected calcium, and ionized calcium had a significant negative correlation with IS with r = −0.578, −0.5396, and − 0.5335, respectively [Figures 1-3]. Pearson's correlation coefficient for serum albumin and IS was not statistically coefficient (r = −0.0463, P = 0.7226). Calcium levels (total, corrected, and ionized) were collapsed into quartiles, and Pearson's correlation coefficient was used for comparing the levels with IS [Table 2]. It can be seen from the table that total and ionized calcium showed a significant negative correlation with IS across all four quartiles. Albumin-corrected calcium levels showed a significant negative correlation with IS only across the lowest and highest quartiles and not the second and third quartiles. In all the three, the correlation with IS was highly significant across the highest quartile of calcium levels (total, corrected, and ionized).
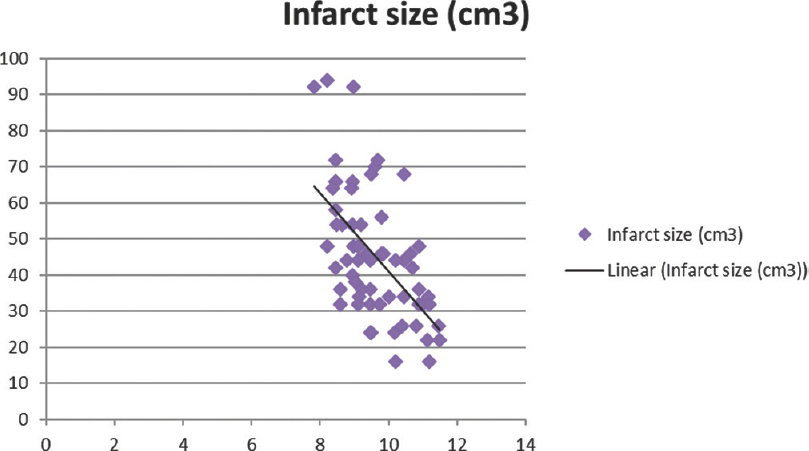
- Significant negative correlation of total calcium with infarct size
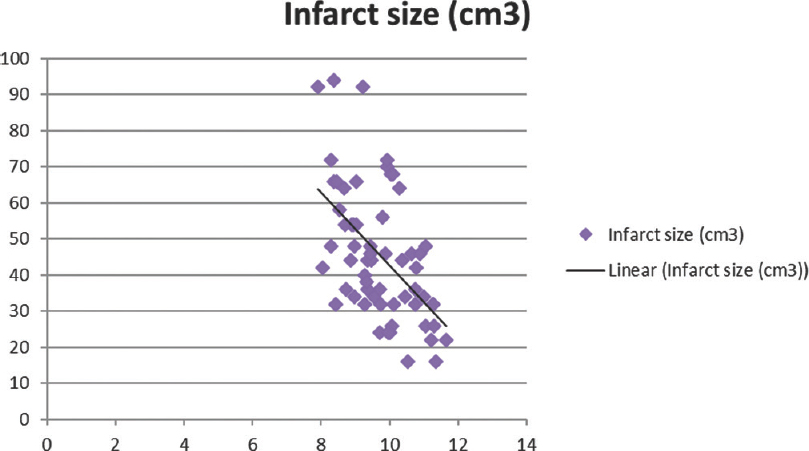
- Significant negative correlation of corrected calcium with infarct size
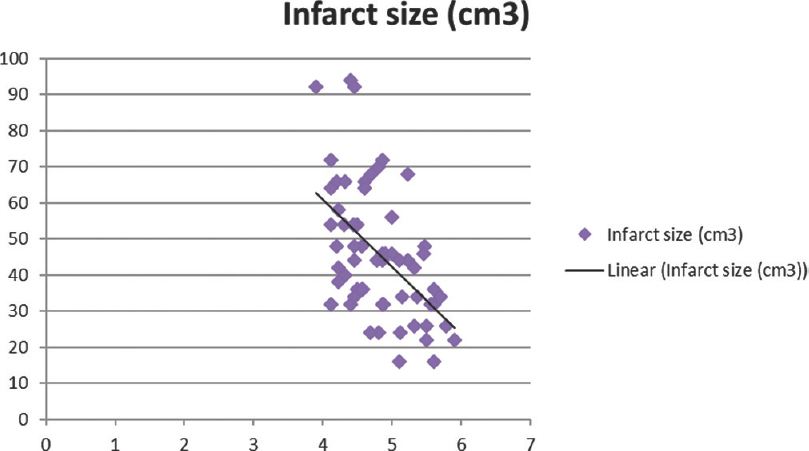
- Significant negative correlation of ionized calcium with infarct size
DISCUSSION
In our study, 61 patients with ischemic stroke were evaluated regarding mainly their serum calcium levels and IS (as measured by CT scan). In the present study, it was found that serum calcium levels had a negative correlation with IS in patients with ischemic stroke, and this correlation was statistically significant. This statistically significant correlation was established for total, albumin corrected, and ionized calcium. These findings were in accordance with those by Kasundra et al.,[18] where a statistically significant (P < 0.05) correlation of IS was found with both calcium level and corrected calcium level. Similar findings were also reported by Buck et al.[16] and D’Erasmo et al.[19] Our study had an added advantage as along with measurement of total calcium and calculation of albumin-corrected calcium levels, ionized calcium was also measured directly.
Hence, our present study is suggestive of the finding that ischemic stroke patients with a larger IS tend to have lower levels of serum calcium and those with smaller IS s tended to have a higher level of serum calcium. The pathophysiological mechanisms underlying the observations of our study are not well defined. However, experimental studies have established that influx of Ca2+ into neuronal cells is a mechanism of ischemic cell death. During ischemia, when the CBF falls below 10 ml/min/100 g, there is an interruption in the oxygen-dependent generation of high-energy compounds. This leads to a disturbance in cellular calcium homeostasis as three of the four mechanisms responsible for maintaining the 10,000:1 (extracellular: intracellular) differential of calcium outside the cells is ATP dependent. A rapid and massive influx of calcium into the cell results.[9] Elevated intracellular Ca2+ activates membrane phospholipases and protein kinases. The integrity of the cell membrane is further damaged due to phospholipases, and this reinforces the failure to maintain calcium homeostasis. As a result more calcium is extracted from the blood into the brain.
For a substantial and noticeable decrease in serum calcium to occur, there must be a complementary increase in the accumulation of intracellular calcium. It is thought that during ischemic stroke, total neuronal cell Ca2+ content may increase to 150% of control or more.[19] The finding that there is a greater decrease in serum calcium in patients with ischemic stroke as compared with patients of transient ischemic attack also lends strength to this hypothesis.[20] Whether this intracellular influx of serum calcium leading to a decrease in the serum calcium, directly correlates with the IS cannot be ascertained with certainty, until direct quantification of intracellular calcium in the ischemic cells can be done.
In our study, Pearson's correlation coefficient was calculated for total, albumin corrected, and ionized calcium. Total calcium had a better correlation with IS as compared to corrected and ionized calcium. Total calcium and ionized calcium had a statistically significant correlation with IS across all four quartiles, but CCa had a statistically significant correlation only with the lowest and highest quartile ranges. This findings are in sync with Ovbiagele et al.,[15] D’Erasmo et al.,[19] and Kasundra et al.,[18] who found total calcium to be a better predictor in the outcome of ischemic stroke as compared to corrected calcium. There was no statistically significant correlation between serum albumin and IS in our study. The correlation of albumin with stroke outcome has been shown to be significant by some and nonsignificant by others.[21222324] However, the occurrence of fluctuations in the levels of serum albumin during acute stroke has also been established without doubt. Our study also found a better correlation with IS across the highest quartile of calcium in all three cases (total, albumin corrected, and ionized). The reason for this also is not exactly known. It is presumably because higher calcium values are associated with smaller ISs, which in itself indicates a lower disturbance in calcium homeostasis. Although calcium from the extracellular compartment moves to the intracellular compartment during ischemia, the degree of rise in intracellular calcium is much more than the drop in extracellular levels.[25]
Our study was not without limitations. First, our study correlated only biochemical (calcium) and radiological (IS) parameters. Functional and clinical indicators such as The National Institute of Health Stroke Scale and Barthel index were not included in the study. In addition, serum calcium level was measured only once, unlike its repeated estimation by Ovbiagele et al.[15] in a similar study. Although care was taken for to carry out the biochemical and radiological investigations immediately after admission, lack of standardization of a fixed time frame may also cause bias in the analysis. However, the annual intraindividual calcium level variation is only ~2%, and it is believed that the degree of change in the levels of serum calcium as noted in our study cannot be completely accounted for by the transport of extracellular calcium into the ischemic neuronal cells.[26]
CONCLUSION
In our study, it was found that total, corrected, and ionized calcium had a statistically significant correlation with IS in ischemic stroke. This correlation was stronger for total and ionized calcium as it was established across all four quartiles of calcium. To the best of our knowledge, this is the first study from Northeast India highlighting this. The findings in our study suggest that serum calcium can be used as a prognostic indicator in ischemic stroke as it directly correlates with the IS. Of particular interest in trying to better determine the potential cause and effect relationship would be to see if serum calcium levels change over time in relationship to IS. Prospective studies designed specifically with a larger sample size and direct measurement of calcium levels at specified time intervals would help in shedding greater light in the exact pathophysiological mechanism of ischemic stroke.
Financial support and sponsorship
The study was supported by North Eastern Indira Gandhi Regional Institute of Health and Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to Dr. Happy Chutia, Dr. Chandan K. Nath, Dr. Kaustubh Bora, Dr. Vinay Upadhyay, and Dr. Binod Gohain for their help during this study.
REFERENCES
- World Health Organization. Preventing Chronic Diseases: A Vital Investment. Geneva, Switzerland: World Health Organization; 2005.
- Ionic changes in brain ischaemia and alterations produced by drugs. Br J Anaesth. 1985;57:23-33.
- [Google Scholar]
- A correlative study of serum calcium levels with Infarct Size in Acute Ischemic Stroke: Observations from Northeast India. Curr Top Membr Transp. 1978;10:151-216.
- [Google Scholar]
- The regulation of intracellular calcium in presynaptic nerve terminals. Ann N Y Acad Sci. 1978;307:195-212.
- [Google Scholar]
- Myocardial ischemia: The pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981;102:271-81.
- [Google Scholar]
- Eicosanoids: Prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. J Neurochem. 1982;38:1-14.
- [Google Scholar]
- Leukocyte-derived metabolites of arachidonic acid in ischemia-induced myocardial injury. Fed Proc. 1987;46:2422-33.
- [Google Scholar]
- Elevated calcium after acute ischemic stroke: Association with a poor short-term outcome and long-term mortality. J Stroke. 2015;17:54-9.
- [Google Scholar]
- Are elevated admission calcium levels associated with better outcomes after ischemic stroke? Neurology. 2006;67:170-3.
- [Google Scholar]
- Association of higher serum calcium levels with smaller infarct volumes in acute ischemic stroke. Arch Neurol. 2007;64:1287-91.
- [Google Scholar]
- ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104-10.
- [Google Scholar]
- Clinico-radiological correlation between serum calcium and acute ischemic stroke. Int J Adv Med Health Res. 2014;1:69-74.
- [Google Scholar]
- Acute serum calcium changes in transient ischemic attack and cerebral infarction. J Med. 1998;29:331-7.
- [Google Scholar]
- Serum albumin at admission for prediction of functional outcome in ischaemic stroke patients. Neurol Sci. 2008;29:445-9.
- [Google Scholar]
- Serum albumin in ischemic stroke patients: The higher the better. The Bergen Stroke Study. Cerebrovasc Dis. 2009;28:13-7.
- [Google Scholar]
- Hypoalbuminemia in acute ischemic stroke patients: Frequency and correlates. Eur J Clin Nutr. 2007;61:1318-22.
- [Google Scholar]
- Serum albumin level as a predictor of ischemic stroke outcome. Stroke. 2004;35:e156-8.
- [Google Scholar]
- Annual rhythmic and non-rhythmic biological variation of magnesium and ionized calcium concentrations. Clin Chem Lab Med. 2001;39:45-9.
- [Google Scholar]






