Translate this page into:
An inexpensive foramen magnum decompression training tool: Feasibility and validation study
*Corresponding author: Claudia L. Craven, Department of Neurosurgery, Cambridge University Hospitals, Cambridge, United Kingdom. claudiacraven@nhs.net
-
Received: ,
Accepted: ,
How to cite this article: Moncur EM, Murphy M, Craven CL. An inexpensive foramen magnum decompression training tool: Feasibility and validation study. J Neurosci Rural Pract. 2024;15:357-60. doi: 10.25259/JNRP_480_2023
Abstract
Foramen magnum decompression (FMD) is a standard neurosurgical procedure, typically utilized to treat Chiari malformation. The aim of this educational project was to develop and validate a low-cost FMD simulation training model. Mold-based methods were used to develop a prototype. Feasibility was tested during an FMD training session for 17 neurosurgery trainees. Face and content validity were assessed through a Likert Scale. The perceived training benefit was determined using the Physician Performance Diagnostic Inventory Scale (PPDIS). A total of 87.5% successfully removed the C1 arch, 81.3% successfully performed an FMD, and 68.8% avoided injury to the underlying structures. The model scored highly for visual and tactile realism. The median confidence rating on PPDIS significantly improved from early learner to competent. We demonstrate feasibility, content, and face validity. Furthermore, this is a low-cost, portable model that can be easily replicated and used for simulation training.
Keywords
Affordable
Validation
Feasibility
Training
Foramen magnum decompression
INTRODUCTION
Foramen magnum decompression (FMD) is a standard neurosurgical procedure. The surgery involves opening the occipital skin and muscle, exposing the occiput and the lamina of the atlas and axis, and using a high-speed drill to remove the C1 arch and lower occiput. The dura over the cerebellum is often opened and the arachnoid below can also be opened if required.
A FMD is utilized to treat Chiari malformation and also for a suboccipital approach. As such, neurosurgical trainees will be expected to learn FMD early on. However, the approach can be daunting, given the presence of critical structures in the region including the vertebral arteries laterally, the torcula superiorly, and deep structures including the cerebellum, medulla, and spinal cord.
Complication rates following FMD can be as high as 20%.[1] One systematic review showed the most frequent complications after FMD are pseudo meningocele (2.7%) and cerebrospinal fluid (CSF) leak (2%) and the overall requirement for revision surgery is around 3%.[2] The aim of this project was to (1) develop a low-cost and replicable model for FMD training and (2) determine the preliminary feasibility and validity of this model.
MATERIALS AND METHODS
Model development
This model was made using mold-based methods, and materials that are readily available from commercial model-making stores. The occiput, foramen magnum, and C1 and C2 vertebrae were cast in silicone mold using Smooth-On© Moldstar™ 30 slow mold (6-h cure time and a shore-A hardness level of 30). Skulls were cast in off-white polyurethane (Polycraft PU resin, MB Fibreglass©) with a cure time of 3 min and Shore-D 72 to mimic bone [Figure 1a]. Before pouring the polyurethane, a small amount of plaster of paris was added (1% of the polyurethane volume poured). The dura mater was made from latex (Polycraft, MB Fibreglass©) painted in three thin layers (around 1–2 mm) inside the occiput. This mimics the durability and eggshell-like peeling of dura. A red dye was added to the liquid latex before painting, to differentiate dura from bone. The latex took 8 h to dry [Figure 1b]. The final model underwent trimming of unintended layers of polyurethane [Figure 1c]. To mimic the spinal cord and cerebellum, with pia mater, grapes were placed within the vertebral canal [Figure 1d].
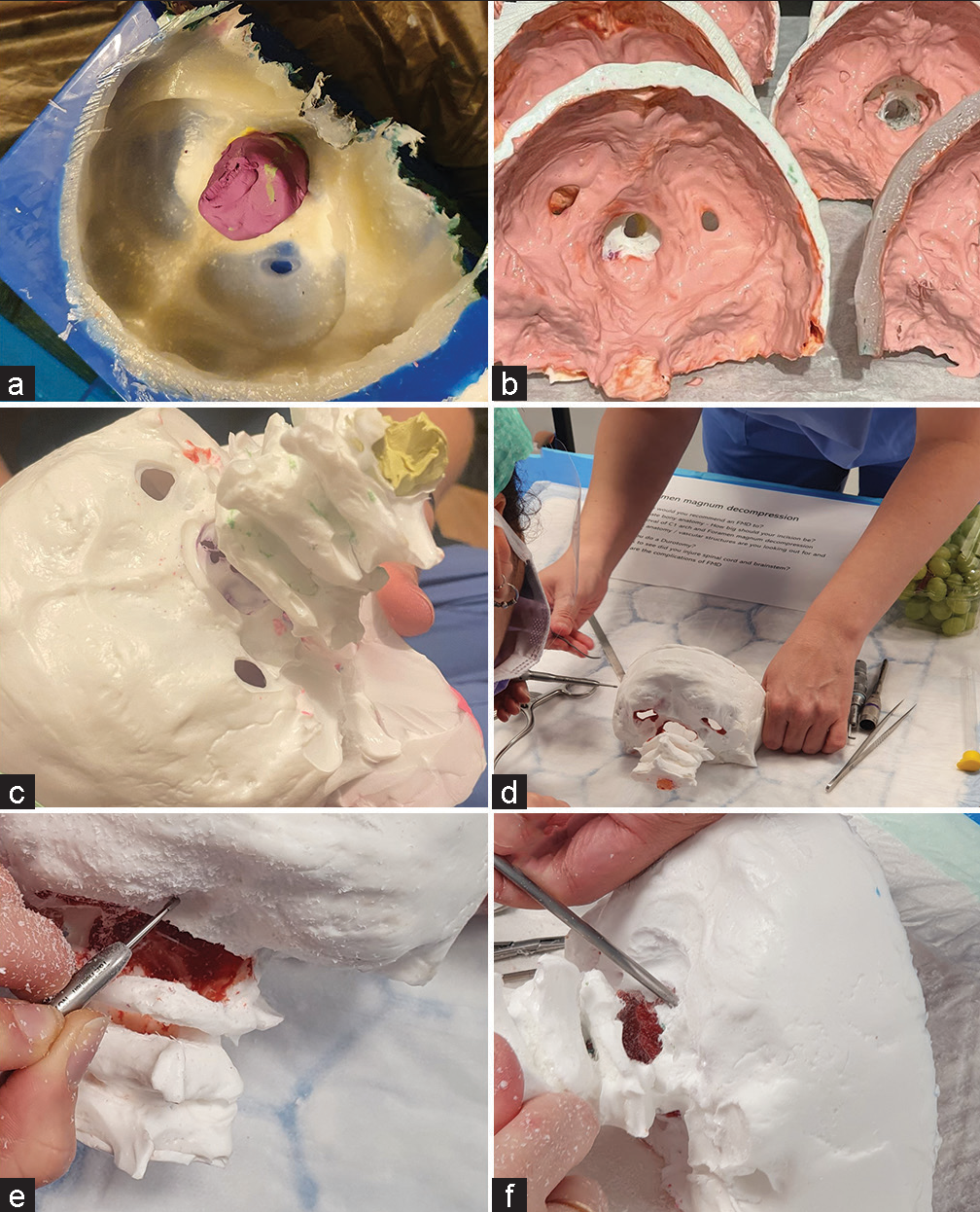
-
(a) The drying polyurethane cast in a silicone mold. (b) The wet latex drying inside the cast occiput. (c) The final model before trimming. (d) The pre-operative posterior view showing the intact occiput with C1–C3 lamina (with grapes ready to insert as a neural issue). (e) Foramen magnum decompression surgery with a high-speed matchstick drill and (f) with Kerrison Rongeur.
Feasibility
The model was trialed at a simulation course for neurosurgical trainees. All individuals were taught by the same three consultant trainers, using specific learning objectives [Table 1]. After training, all participants then undertook an anonymous self-assessment as to how many of the stages they were able to perform.
| Key Stage | Description |
|---|---|
| (1) Landmarks | Palpation of the bone and identification of bony landmarks of the C1 arch, C2 spinous process, the superior and inferior nuchal lines, and the inion. |
| (2) Approach description | A linear midline skin incision from just above the inion to the spinous process of C2. The bilateral paraspinal muscles would be removed using monopolar diathermy. A Y or T-shape fascial (avascular nuchal line) opening to retract the semispinalis capitis and rectus capitis, and retractor placement. Caution to avoid injury to the third occipital nerve and its branches (running in an adipose layer below the obliquus inferior). |
| (3) Removal of the C1 arch | Identification of the posterior arch of C1 and removal with small Kerrison Rongeur (no more than 2 cm on each side), avoiding injury to the underlying dura and nearby vessels. The vertebral artery is just above the C1 arch at the vertebral notch. |
| (4) Foramen magnum decompression | A high-speed drill is used to make burr holes, which can then be connected using a craniotome, or combined with removal with a Kerrison Rongeur. |
| (5) Durotomy | A Y-shape durotomy is made, taking care to avoid injury to the underlying tissue (a grape to mimic neural tissue). To practice opening the arachnoid, a grape can be used. Continuous watertight closure of the dura mater either directly or using a commercial patch and expansion duroplasty was performed. |
| (6) Avoiding injury to the spinal cord and medulla | The grapes were removed and inspected to look for any traumatic injury from the high-speed drill, Kerrison Rongeur, or other instruments. |
Validity
Face validity (the model’s realism) was assessed using anonymized feedback, in the form of a 5-point Likert Scale (1 = highly unrealistic, 2 = quite unrealistic, 3 = undecided, 4 = quite realistic, and 5 = highly realistic). Tactile realism and visual realism were both assessed. Content validity was assessed using anonymized feedback with “Yes”/“No” responses, asking (1) would it be useful for their training and (2) if would they recommend this model to others as a training tool, with free-text to expand on answers.
Physician performance diagnostic inventory scale (PPDIS)
Perceived training benefit was determined using the PPDIS. Learners self-rated their level before and after training on the model, using the PPDIS (1 = unsatisfactory, 2 = early learner, 3 = competent, 4 = proficient, and 5 = expert). A Wilcoxon matched-pairs signed-rank test determined if training resulted in a significant improvement in PPDIS. P < 0.05 was considered significant. All statistical tests were performed on GraphPad Prism 10.0.1.
RESULTS
Demographics
A cohort of 17 delegates mean age 27.8 years (standard deviation ± 3.50) 8M: 9F attended the training course. Two delegates were students, one was a foundation year 1 doctor, four were foundation year 2 or equivalent level, nine were junior specialist training registrars/residents (ST1–ST3) or core trainees, and one was a senior registrar/resident (ST7–8). The majority were early learners, with the exception of one who was proficient.
Course delivery and feasibility
All 17 delegates underwent training on the FMD model, with step-by-step teaching [Table 1]. A total of 81.3% could palpate key bony landmarks, 81.3% were able to describe the approach, 87.5% successfully removed the arch of C1, 81.3% successfully performed an FMD with a drill [Figure 1e] or Kerrison Rongeur [Figure 1f], 50.0% cleanly opened the dura, and 68.8% avoided injury to the underlying structure.
Validation
Visual and tactile realism both scored a median of 4 “realistic” (interquartile range [IQR] 3.5–5). Regarding content validation, 100% of the delegates found the model useful and recommended it to colleagues. Of the 17 delegates, five gave additional free-text answers stating “Absolutely,” “Perfect,” “Very useful and mimics real skull,” “Great,” and “Yes good for workflow and basic concepts.”
PPDIS
Before training on the model, the median PPDIS was 2 (IQR 1–2) which is “early learner.” Following training on the model, this significantly increased to 3 (IQR 2–3), a “competent” trainee (<0.0001) [Figure 2]. Of the 17 learners, none decreased in confidence, five remained the same, 10 improved by 1, and two improved by 2.
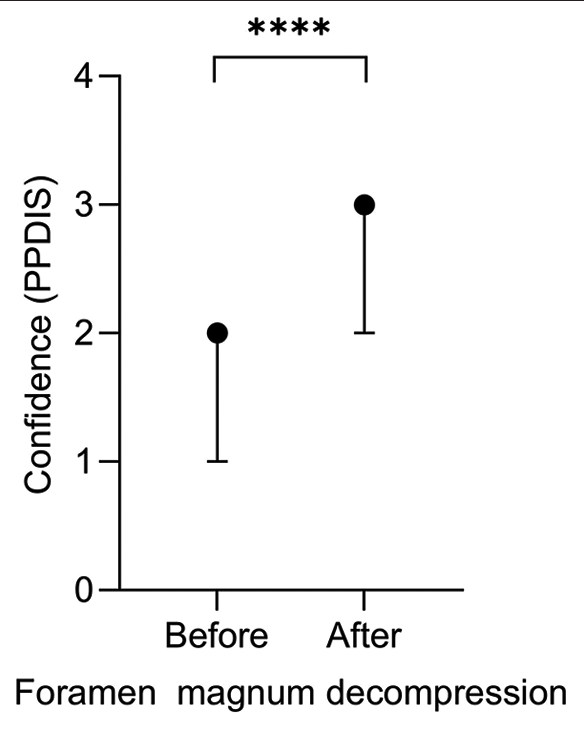
- Median physician performance diagnostic inventory scale before (median 2 = early learner) and after (median 3 = competent) model training with interquartile range, with a significant improvement in confidence after training (<0.0001)****.
DISCUSSION
Key findings
We trialed a low-cost simulation model made specifically for junior neurosurgical trainees to practice the FMD and the suboccipital approach. The model performed well with reasonable visual and tactile realism. Trainees reported it to be a useful training model that they would recommend to others. Furthermore, the model significantly improved learners’ confidence levels in the procedure of FMD.
Effect of COVID-19 on neurosurgery training
Reduced operative exposure following working time directives and increasingly senior-led care has resulted in slower skill acquisition in trainee neurosurgeons.[3] Since COVID-19, the deficit in training has worsened, with trainees having lost over a year of high-volume operating. Post-COVID-19, the rise in waiting lists, time pressures in theater, and emphasis on service provision has further reduced training opportunities.[4]
Simulation and neurosurgery
Simulation allows the trainee to replicate a surgery, without the risks or consequences present in the real world.[5] The traditional apprenticeship style of training is therefore being increasingly supplemented by simulation.[6] In particular, simulation for novices and early learners can be particularly beneficial.[7] Neurosurgery simulators exist in the form of virtual reality, haptic simulators, cadaveric models, animal models, and synthetic models.[6,8,9]
Low-cost simulation models
Physical simulation models have to balance cost with fidelity. This model was developed with the intention of filling a niche for a low-cost, portable, and replicable model for a specific training need. There is a recognized need for models fulfilling these criteria in neurosurgical training.[10] All the materials used are commercially available from high street stores. The total material cost (not including labor or estates) per model was around 20 pounds.
Study limitations
This study focused on early learners of the FMD, with an emphasis on drill training and the use of Kerrison Rongeurs. For this model to be useful for senior trainees, vascular structures and CSF would ideally be added. However, such high-fidelity models already exist. The aim of this model was to be realistic enough for early learners, while also being easily replicable and affordable. As a pilot study mainly assessing feasibility, the cohort size was limited, participants were not timed and repeated measures were not assessed or tested. These measures would be appropriate for future validation studies.
Future developments
The model has the potential to be integrated into courses aimed at junior trainees wishing to practice approaches to the posterior fossa.
CONCLUSION
We demonstrated feasibility, content, and face validity and determined that trainees felt more confident in performing the operation after training on the FMD model. Furthermore, this is a low-cost, portable model that can be easily replicated and used for simulation training.
Ethical Approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Foramen magnum decompression and duraplasty is superior to only foramen magnum decompression in chiari malformation Type 1 associated with syringomyelia in adults. Asian Spine J. 2015;9:721-7.
- [CrossRef] [PubMed] [Google Scholar]
- Post-operative complications after foramen magnum decompression with duraplasty using different graft materials in adults patients with Chiari I malformation: A systematic review and meta-analysis. J Clin Med Res. 2023;12:3382.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of the coronavirus pandemic on European neurosurgery trainees. World Neurosurg. 2021;154:e283-91.
- [CrossRef] [PubMed] [Google Scholar]
- Editorial. Innovations in neurosurgical education during the COVID-19 pandemic: Is it time to reexamine our neurosurgical training models? J Neurosurg. 2020;17:1-2.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of simulation models in neurosurgical training according to face, content, and construct validity: A systematic review. Acta Neurochir (Wien). 2022;164:947-66.
- [CrossRef] [PubMed] [Google Scholar]
- Developing a pediatric neurosurgical training model. J Neurosurg Pediatr. 2018;21:329-35.
- [CrossRef] [PubMed] [Google Scholar]
- The evolution of an SBNS-accredited NANSIG simulated skills workshop for aspiring neurosurgical trainees: An analysis of qualitative and quantitative data. Acta Neurochir. 2020;162:2323-34.
- [CrossRef] [PubMed] [Google Scholar]
- Virtual-augmented reality and lifelike neurosurgical simulator for training: First evaluation of a hands-on experience for residents. Front Surg. 2022;9:862948.
- [CrossRef] [PubMed] [Google Scholar]
- Benchtop simulation of the retrosigmoid approach: Validation of a surgical simulator and development of a task-specific outcome measure score. World Neurosurg X. 2023;20:100230.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical simulation: The way forward or a waste of time? Bull R Coll Surg Engl. 2013;95:288-8.
- [CrossRef] [Google Scholar]







