Translate this page into:
Acute subdural hematoma from a ruptured aneurysm of the distal middle cerebral artery
Address for correspondence: Dr. Dale Ding, Department of Neurosurgery, University of Virginia, P. O. Box: 800212, Charlottesville, VA 22908, USA. E-mail: dmd7q@hscmail.mcc.virginia.edu
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Distal middle cerebral artery (MCA) aneurysms are uncommon cerebrovascular lesions, which frequently have an infectious etiology. Mycotic aneurysms account for < 5% of intracranial aneurysms.[1] Although intracranial hemorrhage is the most common presentation of cerebral aneurysms, acute manifestation of a ruptured aneurysm with a subdural hematoma (SDH) is exceedingly rare.[2] We describe a unique case of a patient with an acute SDH from a ruptured aneurysm of the distal MCA.
A 63-year-old male with a 2-day history of mild headaches was initially found unresponsive. After arrival in the emergency department, the patient awoke confused but had an otherwise nonfocal neurological examination (Glasgow coma scale of 14). Brain computed tomography (CT) showed an 11-mm acute left-sided convexity SDH, and CT angiography (CTA) identified a 6-mm aneurysm of the distal left MCA [Figure 1a–e]. Further evaluation with cerebral angiography found the aneurysm to be arising from the left angular artery, with a morphology and location consistent with a mycotic aneurysm [Figure 1f and g]. The patient was taken emergently to the operating room where a craniotomy was performed to evacuate the acute SDH and obliterate the ruptured mycotic aneurysm. Intraoperatively, the arterial wall of the distal MCA branch harboring the aneurysm was found to be circumferentially affected. The aneurysm wall was very frail, and dissection of the aneurysm resulted in intraoperative rupture. Since the vessel wall was friable, it was unable to be repaired without occluding the parent artery. Therefore, the aneurysm was trapped by applying one clip to each side of the lesion.
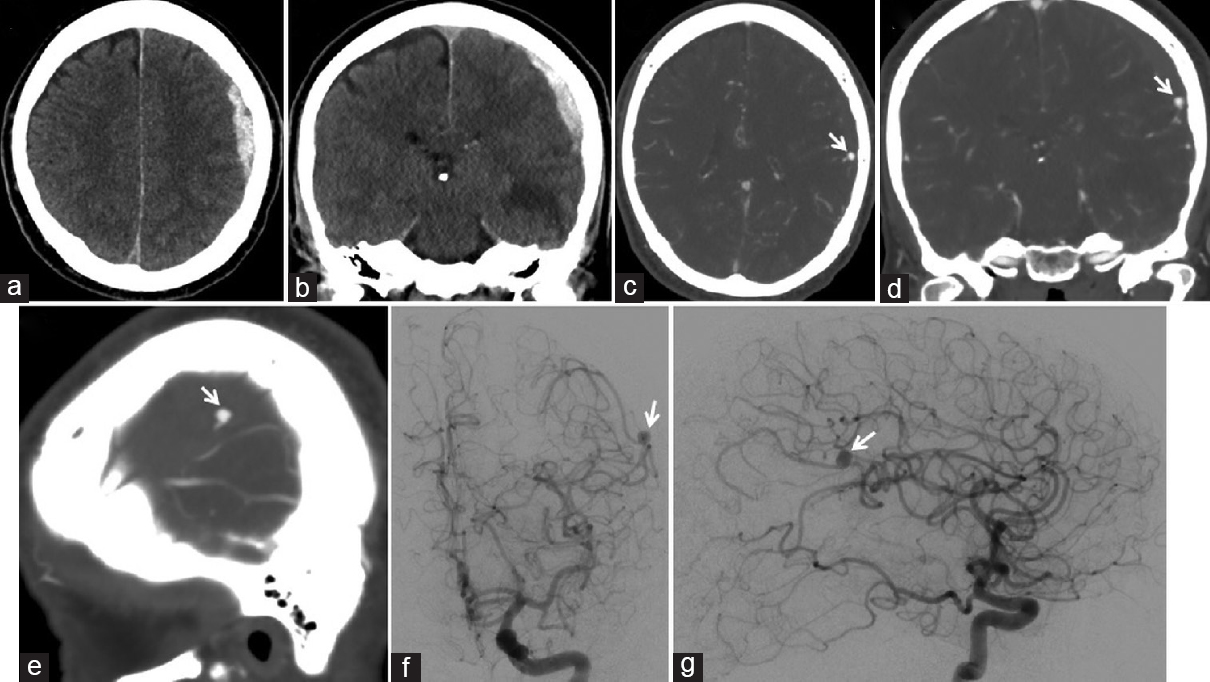
- Preoperative brain computed tomography, (a) axial and (b) coronal views, shows an acute subdural hematoma of the left convexity, measuring 11 mm in maximal thickness, resulting in 3 mm of midline shift. Preoperative brain computed tomography angiography, (c) axial, (d) coronal, (e) and sagittal views, shows a ruptured 6 mm × 5 mm aneurysm (arrow) arising from the distal left middle cerebral artery. Preoperative cerebral angiography, (f) anteroposterior and (g) lateral views of a left computed tomography angiography injection, shows an aneurysm (arrow) arising from the distal left angular artery, favoring a mycotic aneurysm based on its location and morphology
The patient had an uncomplicated postoperative course and was transferred to an inpatient rehabilitation facility 2 weeks after surgery. At the time of transfer, the patient was ambulating independently with no focal neurological deficits but was noted to have poor cognition, attention, and information processing. A complete infectious workup, including a transesophageal echocardiogram, did not identify a causative organism for the mycotic aneurysm. Based on the recommendations of the consulting infectious diseases team, the patient was administered a 4-week postoperative course of intravenous flucloxacillin. Postoperative brain CTA at 2 months follow-up showed no evidence of a recurrent or de novo aneurysm [Figure 2].
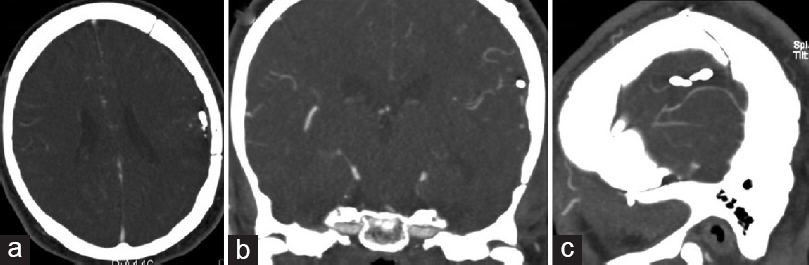
- Postoperative brain computed tomography angiography performed 2 months after craniotomy for trapping of the mycotic aneurysm, (a) axial, (b) coronal, (c) and sagittal views, shows no evidence of a recurrent or new aneurysm
MCA aneurysms most commonly arise from the bifurcation and less frequently from the M1 segment at the origin of the anterior temporal artery branch. Distal MCA aneurysms of the M3 and M4 segments are considerably less prevalent and are morphologically distinct from proximal MCA aneurysms. Specifically, distal aneurysms tend to be fusiform appearing as circumferential dilatations of the affected artery, whereas the majority of proximal aneurysms are saccular. Due to these differences, aneurysms in these two locations require distinct treatment considerations. Saccular aneurysms of the proximal MCA can be obliterated with surgical clipping or endovascular coiling. In contrast, since the entire arterial segment of a fusiform aneurysm is diseased, distal MCA aneurysms may require surgical trapping, with or without excision and bypass (e.g., for arteries supplying eloquent brain areas, such as sensorimotor and language cortex) or endovascular stenting (e.g., flow diversion). These aforementioned strategies can be challenging to employ due to the small caliber of the distal vessels.
Presentation of a distal MCA aneurysm with an acute SDH is very unusual, and only a handful of similar cases have previously reported in the literature.[2] Aneurysms can, in rare instances, rupture through the dura, thereby resulting in an acute SDH. Aneurysms in proximity to dural surfaces, such as M4 segment MCA aneurysm in our patient, are more prone to incur SDH. Compared to aneurysmal subarachnoid hemorrhage, which is frequently diffuse, aneurysmal SDH is a focal lesion which can be extensively evacuated at the time of surgical aneurysm intervention. Although one could hypothesize that vasospasm may be less common after aneurysmal SDH, the paucity of aneurysmal SDH cases in the literature impairs a rigorous comparison of the two presentations of aneurysm rupture.
Since the patient's aneurysm was not excised in our case and intraoperative cultures of the affected arterial segment were not obtained, we lacked histopathological evidence for an infectious etiology of the lesion. In addition, the lack of multiple aneurysms and the absence of cardiovascular vegetation suggest that the lesion may not have been a mycotic aneurysm. However, based on the angiographic and intraoperative findings, we believe the aneurysm was likely mycotic. In our patient, we decided to further investigate his SDH with vascular imaging, which was initially a CTA, due to the spontaneous onset of the SDH and the patient's lack of known coagulopathy. Although we did not specifically suspect an aneurysmal source for the patient's SDH at the time of presentation, our intent in performing the CTA was to rule out an underlying vascular lesion. In our case, catheter angiography was performed after the initial CTA identified an aneurysm. We recommend noninvasive vascular imaging, either CTA or magnetic resonance angiography (MRA), for patients presenting with a spontaneous SDH in the absence of a history of coagulopathy. However, since dural arteriovenous fistulas and small pial arteriovenous malformations can be missed on CTA or MRA, particularly in the setting of acute hemorrhage, we believe that six-vessel catheter cerebral angiography is warranted in the vast majority of cases of spontaneous SDH.
Mycotic aneurysms are fragile lesions which are prone to rupture. While unruptured mycotic aneurysms can be medically managed with antibiotic therapy, ruptured mycotic aneurysms generally require treatment with surgical or endovascular intervention.[3] Antibiotic therapy is most effective when a causative organism is isolated. In patients with unruptured mycotic aneurysms in whom a causative organism cannot be identified, empiric medical treatment with broad-spectrum antibiotics is a reasonable option. However, patients harboring mycotic aneurysms who are managed with antibiotic therapy alone require close angiographic surveillance for aneurysm regression. Unfortunately, in our case, the follow-up duration of 2 months was relatively brief. Long-term clinical and angiographic follow-up is necessary for patients treated with antibiotics, surgery, or embolization to exclude mycotic aneurysm recurrence or de novo formation.
Unlike the pathogenesis of classic saccular intracranial aneurysms, a mycotic aneurysm's parent artery is circumferentially diseased, most commonly due to bacterial infiltration of the vessel wall.[45] As such, parent artery preservation is generally not possible during the treatment of these lesions. However, due to the distal location of most mycotic aneurysms, parent vessel occlusion can typically be tolerated without causing a neurological deficit, such as in our case. Although rarely necessary, revascularization is particularly challenging due to the small caliber and frail walls of the affected arteries. Patients with a ruptured mycotic aneurysm and a concurrent acute SDH should be preferentially managed with surgery, which can simultaneously treat both lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Intracranial infectious aneurysms: A comprehensive review. Neurosurg Rev. 2010;33:37-46.
- [Google Scholar]
- Ruptured mycotic aneurysm of the distal middle cerebral artery manifesting as subacute subdural hematoma. J Cerebrovasc Endovasc Neurosurg. 2013;15:235-40.
- [Google Scholar]
- Endovascular stenting for treatment of mycotic intracranial aneurysms. J Clin Neurosci. 2014;21:1163-8.
- [Google Scholar]
- Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res. 2014;5:338-46.
- [Google Scholar]
- Critical role of TNF-a in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77.
- [Google Scholar]





