Translate this page into:
A striking mimic: Severe cytomegalovirus polyradiculitis resembling anterior horn cell disease in an immunocompetent woman
*Corresponding author: Divyani Garg, AIIMS, New Delhi, India. divyanig@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rani D, Singla D, Agarwal A, Garg D. A striking mimic: Severe cytomegalovirus polyradiculitis resembling anterior horn cell disease in an immunocompetent woman. J Neurosci Rural Pract 2023;14:759-61.
Dear Editor,
The etiological differential for motor neuron involvement ranges from treatable conditions to degenerative disorders. The diagnosis of motor neuron disease (MND) always carries multitudinous diagnostic possibilities that must be meticulously evaluated for. We report a patient with severe cytomegalovirus (CMV) polyradiculitis as a mimic of lower motor neuron (LMN) type of MND.
A 42 years-old female initially presented to an outside center with left upper limb weakness developing over 10 days, 40 days back. She initially developed flaccid weakness of the left medial two fingers, followed by weakness of all fingers, and then difficulty in raising the left upper limb. After 1 month, she noted weakness of the left foot with difficulty in gripping slippers, with left lower limb complaints evolving over 9 days. Although there were no sensory complaints, she had moderate pain in both limbs. There was no cranial nerve or autonomic involvement. On examination, she had hypotonia of left upper and lower limbs. Power examination showed power Medical Research Council grade 0/5 in all muscle groups of the left upper limb except shoulder flexion which was 3/5. Left lower limb power grading was 4–/5. Reflexes were attenuated on the left side. Plantar response was flexor. Other neurological and systemic examination was normal. No fasciculation or atrophy was observed at this timepoint. Evaluation showed pure motor axonal neuropathy of the left median, ulnar, radial, peroneal and tibial nerves on nerve conduction studies (NCSs), without conduction block. Electromyography (EMG) showed neurogenic involvement of left C5-T1 and L2-L3 segments without spontaneous activity. Magnetic resonance imaging (MRI) brain and spine were normal. Cerebrospinal fluid (CSF) examination showed 1 cell, sugar 119/blood sugar 190 mg/dL, protein 71 mg/dL. CSF gram stain, bacterial culture, fungal smear, acid-fast bacteria smear, India ink, cryptococcal antigen, venereal disease research laboratory and GeneXpert for tuberculosis and malignant cytology were negative. She was administered intravenous immunoglobulin for 5 days, with a presumptive diagnosis of a chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) variant. Antiganglioside antibodies, autoimmune panel, paraneoplastic panel and positron emission tomography-computed tomography whole body were normal. She was also started on weight-based oral steroids and azathioprine 100 mg. At 1 month of follow-up, the patient continued to deteriorate with symptoms developing on the right side. At this point, she presented to our center.
Examination showed worsening power in the left lower limb (2/5) and development of weakness in the right distal upper limb, with grip strength of 40%. Right lower limb proximal power was 3/5 (hip), 3/5 (knee) and ankle power was 1/5. She had generalized areflexia and generalized muscle atrophy. She had an immobile tongue with atrophy and fasciculations [Figure 1]. She was tachypneic with type 2 respiratory failure, for which she was ventilated. Repeat NCS and EMG at our center showed pure motor axonal changes in all limbs (left>right) and neurogenic changes with occasional fasciculation in the cervical, bulbar and lumbosacral segments. MRI of the brachial and lumbosacral plexus showed polyradicular thickening with enhancement [Figure 2a and b]. CSF was repeated and showed 15 cells, protein 94 mg/dL and normal sugar. CSF real time-polymerase chain reaction (RT-PCR) for neurotropic virus panel was positive for CMV. The patient was initiated on injectable ganciclovir 250 mg twice daily, and steroids and azathioprine were discontinued. Human immunodeficiency virus (HIV) serology and CD4 counts were normal. Fundus examination was normal. Blood CMV RT-PCR showed 820 copies/mL. CSF was repeated after 2 weeks of ganciclovir and showed persistent CMV positivity. She was continued on ganciclovir. However, during this course, she succumbed to severe sepsis.
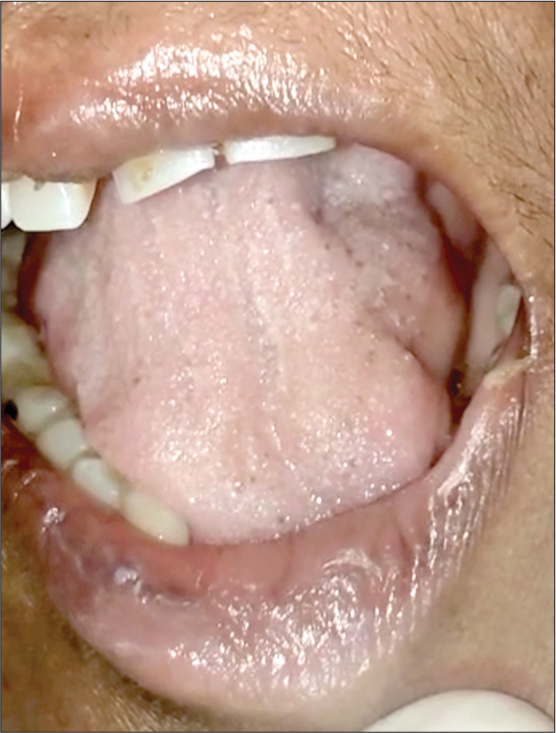
- Atrophy of the genioglossus muscles.
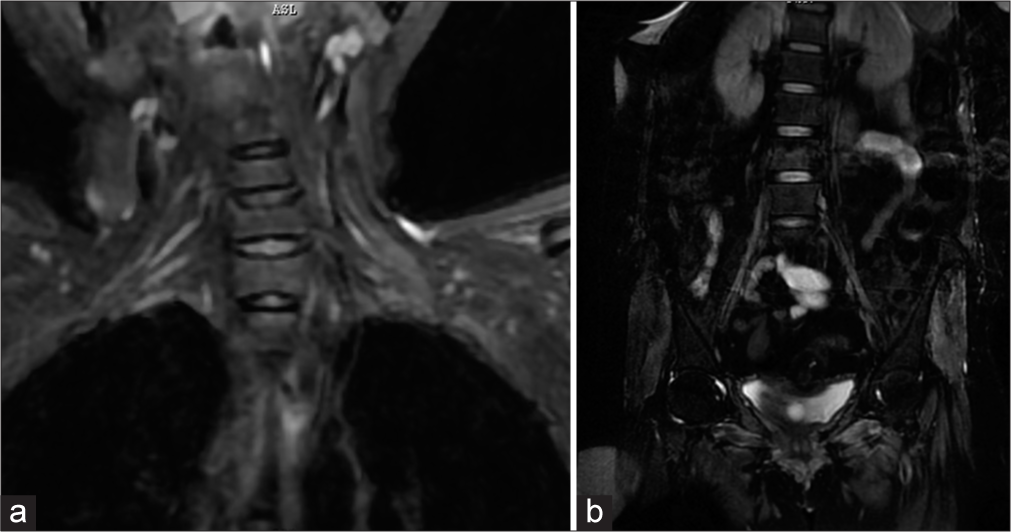
- Magnetic resonance imaging short tau inversion recovery coronal images showing (a) Thickening and hyperintense change of bilateral cervical roots (Left>Right) and (b) Thickening and hyperintense signal change in bilateral lumbosacral roots.
We report severe CMV polyradiculitis as a mimic of LMN-type of MND in an otherwise immunocompetent patient. Clinical clues to consider a mimic included ultra-rapid progression, pain at onset, and remarkable paucity of limb fasciculations despite significant wasting. Mimics of LMN-only MND include multifocal motor neuropathy with conduction block, spinobulbar muscle atrophy, motor-dominant CIDP, inclusion body myositis, and rarely, polyradiculopathies, including compressive, radiation-induced and infection-related.[1] Among infective polyradiculopathies reported to produce a MND-like illness, foremost is HIV,[2-5] a potentially reversible cause.[2] Whereas most reports describe LMN-only syndrome, hyperreflexia may occur, mimicking amyotrophic lateral sclerosis (ALS).[3-5] Among other viral agents, there is one case report of an ALS-like illness associated with Chikungunya infection.[6]
Classically, CMV polyradiculitis, which also frequently involves the lower spinal cord concomitantly, occurs in HIV-affected patients, with subacute onset lower limb weakness, sensory and bladder involvement. This syndrome has been uncommonly reported in immunocompetent patients.[7,8] Other neurological manifestations of CMV infection include meningitis, encephalitis, cranial and peripheral neuropathy. Antiviral therapy is usually prolonged, and resolution may only be partial. The patient had a phenotype of areflexic asymmetric involvement with motor component in electrophysiology with tongue fasciculations which could be very well seen in immune mediated polyneuropathy which also can have tongue fasciculations. However, CSF PCR is a highly sensitive test for CMV detection with sensitivity up to 90% and specificity of up to 96%.[9]
Our case represents an extremely rare case of CMV-related polyradiculopathy that led to a clinical suspicion of anterior horn cell disease due to a rapid course, profound wasting, and tongue fasciculations. It is still possible that CMV eventually involved the anterior horn cells in our patient, albeit starting with radicular involvement, although we did not evince much evidence for anterior horn cell involvement clinically, electrophysiologically or radiologically. This case serves as a reminder to consider the possibility of infective polyradiculopathy as a mimic of MND, in patients with an LMN-only presentation.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Mimics and chameleons in motor neurone disease. Pract Neurol. 2013;13:153-64.
- [CrossRef] [PubMed] [Google Scholar]
- Reversible ALS-like disorder in HIV infection. Neurology. 2001;57:995-1001.
- [CrossRef] [PubMed] [Google Scholar]
- An ALS-like syndrome with new HIV infection and complete response to antiretroviral therapy. Neurology. 2001;57:1094-7.
- [CrossRef] [PubMed] [Google Scholar]
- Amyotrophic lateral sclerosis-like presentation in a HIV-positive patient. J Int Assoc Provid AIDS Care. 2014;13:515-8.
- [CrossRef] [PubMed] [Google Scholar]
- ALS-like disorder in three HIV-positive patients: Case series. Case Rep Neurol. 2021;13:59-64.
- [CrossRef] [PubMed] [Google Scholar]
- Amyotrophic lateral sclerosis-like syndrome after chikungunya. Cureus. 2019;11:e5876.
- [CrossRef] [Google Scholar]
- Elsberg syndrome secondary to Cytomegalovirus infection in an immunocompetent patient: A case report. Neurol Neuroimmunol Neuroinflamm. 2023;10:e200079.
- [CrossRef] [PubMed] [Google Scholar]
- Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol J. 2008;5:47.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of Cytomegalovirus infections. Infect Disord Drug Targets. 2011;11:466-74.
- [CrossRef] [PubMed] [Google Scholar]





