Translate this page into:
A Case of Acute Paraplegia Due to Aortic Dissection in Marfan Syndrome
Address for correspondence: Dr. B. Prakash, Kasturi Neuro Diagnostic Centre, 89-A, East Lokamanya Street, R.S. Puram, Coimbatore - 641 002, Tamil Nadu, India. E-mail: prakashneuro@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
A 45-year-old woman went to bed well by 10 pm after finishing strenuous household works. She woke up around 4 am due to chest and back discomfort with sweating. She could not turn over or sit-up. The lower limbs were felt cold, numb, and weak. The emergency department physician had suggested X-ray of the spine to check for compressive pathology. She had cardiac arrest at the radiology department, where she was revived and shifted to intensive care unit, sedated, and intubated. After 6 h of stabilization, she could be extubated. There was no history of fever, diarrhea, trauma, visual impairment, chest pain, palpitation, or dyspnea preceding the event. However, she had episodes of unexplained chest discomfort at rest for the previous 2 weeks. There was no other significant medical history. Clinically, she was conscious and afebrile, but was in discomfort due to back pain, and could not lie down properly. She could not describe her problems and was not able to localize the tender spot in the spine. Her peripheral pulses were well felt in all the four limbs. The pulse rate was 76/min, and blood pressure was 140/50 mm of Hg, with a collapsing pulse in the upper limbs. Early diastolic murmur was made out by auscultation. Respiratory system and abdominal examination were normal. Neurologically, cranial nerve and upper limb examinations were normal. No Horner's syndrome was noted. She had weakness of Medical Research Council grade 0/5 in both lower limbs from hip downward with hypotonia. Deep tendon reflexes in the upper limbs were brisk, which could not be elicited in lower limbs. She was able to describe the absent pinprick below her D-10 dermatome but could not cooperate well for posterior column examination. Plantar reflexes were absent on both the sides. In view of the above findings, the clinical diagnoses of myocardial infarction (MI), aortic regurgitation (AR), with anterior spinal artery (ASA) embolism causing paraplegia were made. Aortic disease was not considered initially as the peripheral pulses were normal. The patient did not have typical pain of dissection. Early diagnosis of Aortic Dissection (AD) at critical setup is often difficult.[1] Furthermore, paraplegia as a presenting manifestation of AD is rare.[2] Other differential diagnoses of spinal arteriovenous malformation, dural arteriovenous fistula, epidural abscess, and spinal cord demyelination were also considered. However, a single consolidated diagnosis which explains all the findings Could not be arrived at this point. The patient was subjected to investigations. X-ray of the dorsal spine did not reveal vertebral collapse or other abnormalities. Her routine blood investigations, liver function tests, and electrolytes were normal. Electrocardiogram showed anterior wall MI. The troponin-T level was high (80.97 pg/ml). Her echocardiogram showed mild left ventricular dysfunction, global hypokinesia, ejection fraction of 45%, mild AR, mild mitral regurgitation with dissection flap in ascending and arch of aorta extending into descending aorta. Three attempts of doing magnetic resonance imaging (MRI) went in vain as she could not lie down for more than few minutes and not sedated in view of hemodynamic instability. However, we could complete computed tomography (CT) angiogram of the aorta which revealed a Stanford-A, de-Bakey type-1 dissection of the aorta with entry point at the coronary sinus, extending upward to involving root, arch of aorta, descending thoracic and abdominal aorta extending into both common iliac and proximal external iliac arteries. All the three major branches of arch of aorta were involved. The true lumens of left main, right coronary arteries were compressed and small throughout their length. The left renal artery was arising from the true lumen at the site of narrowing [Figure 1]. Patient was advised to undergo emergency surgery, under high life risk with poor outcome of paraplegia, which was explained in detail and her attendees agreed after prolonged counseling. The operation was performed through a midline sternotomy. Cardiopulmonary bypass was achieved. The aortic dissection was found to have originated in the right coronary sinus in close relation to the right coronary ostium. The left main coronary ostium looked clear without any obstructions. Under moderate hypothermia, the aortic root was replaced with a St. Jude Medical valved Dacron conduit. A reversed saphenous vein graft was sutured to the right coronary artery and connected proximally to the aortic graft. The distal aorta was anastomosed to the Dacron graft. The patient was weaned off bypass with stiff ionotropic support and shifted to the intensive care unit. She made slow but steady recovery with good hemodynamics, with no fresh focal neurologic deficit and gradual improvement in creatinine. She was transferred to the postoperative ward on day 8 and fit for discharge on the 14th postoperative day. Despite adequate physiotherapy, medial, nursing, and nutritional care, she had developed sacral sores and the sensory level had ascended to D-2 dermatome. There was no improvement in lower limb power. She also continued to have double incontinence. A CT scan of the brain done on the 5th postoperative day did not show any abnormality. The marfanoid features were noted later after the diagnosis of AD was made. She had wrist sign, thumb sign, probable malar hypoplasia, and arm span-height ratio being 171:165 (>1.04) [Figure 2]. The patient's only son also had marfanoid features. The dissected aorta was sent for histopathology. The gross sample was 2 cm in length and 3 cm in diameter. Microscopy revealed focal loss of elastic and mucoid material of media. There are areas of disruption of elastic laminae replaced by smooth muscle, mucoid material, and collagen. The above features suggest aortic dissection secondary to cystic medial degeneration of ascending aorta [Figure 3].
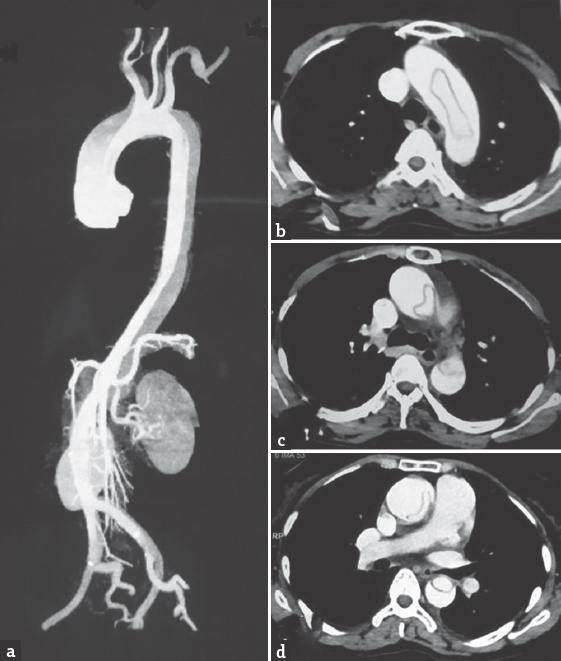
- Computed tomography angiogram of the aorta. (a) Dissection of the aorta from the root involving carotids, renal artery up to iliac vessels. (b) True lumen inside the arch of the aorta. (c) Sausage-shaped true lumen in descending aorta. (d) Further reduction in the size of true lumen
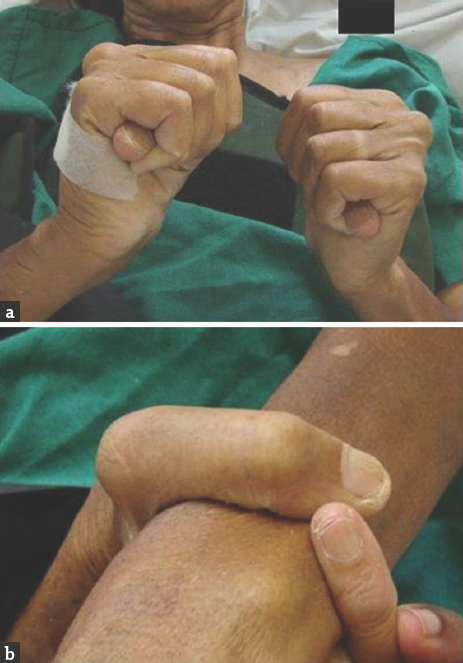
- Clinical features of Marfan syndrome. (a) Thumb sign. (b) Wrist sign
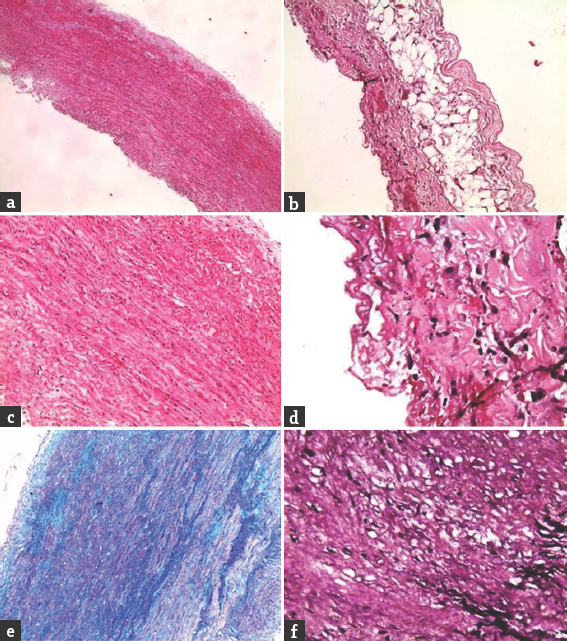
- Histopathology of the dissected aorta. (a) Normal vessel wall. (b) Periadventitial adipose tissue. (c) Vessel wall showing mucoid material. (d) Inflammatory cells. (e) Alcian blue/periodic acid–Schiff stain showing mucoid material. (f) Verhoeff's-Van Gieson showing loss of elastic laminae (in black)
DISCUSSION
Acute AD is a cardiothoracic emergency and acute paraplegia is neurological emergency. The diagnostic difficulties encountered in our patient include poor description of t the diagnosis he pain due to her restlessness as she had recent cardiac arrest, missing marfanoid features clinically due to supine position and sickness. Normal peripheral pulses and the inability to perform MRI also had made the diagnosis difficult. Embolic spinal cord infarction was initially suspected due to the occurrence of simultaneous MI with elevated troponin-T. Echocardiogram had given the first clue about aortic dissection. Unfortunately, MRI of the spine could not be done post operatively also due to recent surgery and staples. This case was presented due to its rare combination of paraplegia due to spinal cord infarction, caused by aortic dissection, secondary to Marfan syndrome associated with acute MI and cardiac arrest, without compromising carotid and renal circulations, and to share the difficulties encountered in making a prompt initial diagnosis. The clinical features of Marfan syndrome include ectopia lentis, joint laxity, dolichostenomelia, scoliosis, and pectus deformities. Retinal detachment, early cataract, and glaucoma are other features. The life expectancy is reduced in severe forms who have greater diameter of aortic root and arch (60%). If the difference in aortic root diameter is more than 5 mm in a patient with family history, prophylactic aortic root replacement should be considered. The aortic dissection is one of the major Ghent criteria[3] for the diagnosis of Marfan syndrome. Fibrillin-1 gene mutation at chromosome 15q-12.1 is believed to play an important role in the pathogenesis of Marfan syndrome.[4] Vascular etiology should always be considered in acute paraplegia. ASA infarction is the major cause for this. Classical clinical features of ASA infarction include acute onset of paraplegia with a pain sensory level but preserved posterior column sensation as the posterior column is supplied by a pair of posterior spinal arteries with multiple collaterals. ASA infarction is often due to the involvement of artery of Adamkiewicz, which arises from posterior aorta.[5] The origin of this artery varies, either from an inferior intercostal (T6–T12) or from superior lumbar artery (L1–3) and mostly on the left side.[1] The simultaneous occurrence of paraplegia and aortic dissection by themselves cannot confirm the correlation between the two, and no criteria exist to prove their relationship.[1] The AD might be considered an incidental finding if the patient did not have an acute MI, cardiac arrest, or other evidence of acute AD at the time of presentation. The AD should be assumed acute if there is acute paraparesis with one of the following features such as chest pain, migratory pain, pulse deficit, aortic insufficiency murmur, cardiac tamponade, stroke, altered consciousness, or aortic bruits.[6] In AD, the dissecting blood flow stretches, narrows, occludes, and later causes tearing of intercostal arteries, with spinal cord infarction. The other hypothesis is the spasm of the intercostal artery and temporary obstruction of the orifice.[1] Apart from paraplegia at the time of presentation, there are reports of perioperative (5%–40%) and postoperative paraplegia on repair of AD.[7]
The annual incidence of AD is 27–32 per million,[28] bearing high mortality (80%).[15] The high-risk population includes the patients with advanced age, chronic hypertension (70%–80%), Marfan syndrome (5%–9%), Turner's syndrome, Ehlers–Danlos syndrome, aortic aneurysm, annuloaortic ectasia, aortic arch hypoplasia, coarctation of aorta, bicuspid aortic valve (7%–14%), connective tissue disorders, cocaine abuse, and atherosclerosis.[8] The symptomatology depends on arteries and organs involved varying from stroke (5%), MI (10%), limb ischemia (20%), renal infarction (15%), spinal cord and mesenteric ischemia (3%).[2] Neurological consequences of AD are due to cerebral, spinal cord or peripheral nerve ischemia.[5] Spinal cord infarction as a manifestation of AD is rare (2%–8%).[9] AD with and without typical pain (5%) causing paraplegia with various degrees of improvement were presented from all over the world.[1] If there is no typical pain, there are high chances of missing the diagnosis.[25] In painless AD, neurological symptoms such as altered mental state, limb numbness, hemiparesis, paraparesis, and Horner's syndrome will provide a diagnostic clue.[1] The possible explanations of painless dissection include reentry of dissected hematoma into true lumen, involvement of carotids making the patient drowsy and loss of visceral and spinothalamic perception of pain due to complete spinal cord infarction.[10] Either with or without the diagnosis, if emergency repair was not undertaken, the prognosis would be poor.[2] Mortality of untreated aortic dissection increases by 1%–2% per hour with a survival rate of 40% at 24 h and 10% at 72 h.[8] Even with timely surgery, the risk of mortality falls on the higher side depending on the vessels occluded and the severity of dissection.[5] Cardiac tamponade is reported in AD by many authors as the cause of death.[6] The patient presented to Adam et al. had painful dissection presenting as paraplegia, with CT confirmation of AD type Stanford-B, and was treated conservatively by cerebrospinal fluid drainage for 3 days, which helps by improving the spinal cord perfusion pressure.[9] However, no controlled trials are available.
CONCLUSION
Aortic dissection in emergency settings should be considered in cases of acute paraplegia[2] and a high index of suspicion is a must.[8] Pain need not always be present. Although the outcome of paraplegia is known to be poor, immediate surgical correction of dissected aorta is mandatory to save other organs and the life of the patient. The mortality rate will increase if there is delay in diagnosis.[11] General physical and all systemic examination are mandatory even in emergency settings, which may give important clues for the accurate and complete diagnosis. Occurrence of pressure sore in absent sacral sensation is common and higher level of care must be executed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Paraparesis as the major initial presentation of aortic dissection: Report of four cases. Acta Neurol Taiwan. 2004;13:192-7.
- [Google Scholar]
- Acute aortic dissection presenting as painless paraplegia: A case report. J Med Case Rep. 2016;10:99.
- [Google Scholar]
- Revised Ghent criteria for the diagnosis of Marfan syndrome (MFS) and related conditions. J Med Genet. 2010;47:476-85.
- [Google Scholar]
- Acute Paraplegia as the Presentation of aortic dissection – A case report. Tzu Chi Med J. 2005;17:369-71.
- [Google Scholar]
- Acute paraplegia: A presenting manifestation of aortic dissection. Am J Med. 1988;84:765-70.
- [Google Scholar]
- Acute postoperative paraplegia complicating with emergency graft replacement of the ascending aorta for the type A dissection. Ann Thorac Cardiovasc Surg. 2003;9:330-3.
- [Google Scholar]
- Aortic dissection-induced acute flaccid paraplegia treated with cerebrospinal fluid drainage. Autops Case Rep. 2012;2:25-8.
- [Google Scholar]
- Aortic dissection presenting primarily as acute spinal cord damage: A case report and literature review. J Int Med Res. 2012;40:2014-20.
- [Google Scholar]





