Translate this page into:
Recurrent post-operative extradural and subdural collections due to intracranial hypotension following a lumbar subarachnoid drain
*Corresponding author: Krishna Prabhu, Department of Neurosurgery, Christian Medical College, Vellore, Tamil Nadu, India. krishnaprabhu@cmcvellore.ac.in
-
Received: ,
Accepted: ,
How to cite this article: Goyal-Honavar A, Joseph J, Jonathan GE, Prabhu K. Recurrent post-operative extradural and subdural collections due to intracranial hypotension following a lumbar subarachnoid drain – A case report. J Neurosci Rural Pract. 2024;15:134-6. doi: 10.25259/JNRP_320_2023
Abstract
Intracranial hypotension (IH) represents a syndrome secondary to low cerebrospinal fluid pressure. This case of IH following a lumbar drain inserted before the excision of a left intraconal lesion, leading to recurrent post-operative unilateral subdural and extradural collections, was treated successfully with the evacuation of the collection and simultaneous epidural blood patch (EBP) injection. Our report provides an important perspective on the management of IH with recurrent intracranial collections and reiterates that IH should be considered when dealing with recurrent unilateral intracranial collections in the post-operative period. Evacuation with a simultaneous EBP is an effective strategy for managing IH.
Keywords
Intracranial hypotension
Recurrent
Collection
Lumbar drain
Case report
INTRODUCTION
Intracranial hypotension (IH) is a clinical syndrome resulting from low CSF pressure; Spontaneous and iatrogenic IH are common, with classical clinical features and serious but rare complications of chronic bilateral subdural hematomas (SDH). Descriptions of IH in the postoperative setting are sparse, especially those presenting with recalcitrant extradural and subdural collections. In this report, we describe an atypical case of postoperative IH, with good long-term outcome following evacuation of the collection with an epidural blood patch in the same sitting.
CASE REPORT
This 41-year-old gentleman presented to our neurosurgical service with loss of vision in the left eye for 1 month, with severe throbbing pain and numbness over the V1 distribution. Examination revealed a fixed, dilated left pupil with ptosis and complete ophthalmoplegia. He was unable to perceive light in his left eye, and the left corneal reflex was abolished. Fundus copy revealed primary left optic atrophy. The facial sensation was reduced by 50% along the left V1 distribution. Magnetic resonance imaging (MRI) of the brain revealed a T2w-hyperintense lesion occupying the superior portion of the left cavernous sinus [Figure 1a], extending into the intraconal space. It measured 4.3 cm in maximum dimension [Figures 1b and c], demonstrating heterogeneous contrast enhancement [Figure 1d]. This clinicoradiological picture suggested a V1 nerve sheath tumor.
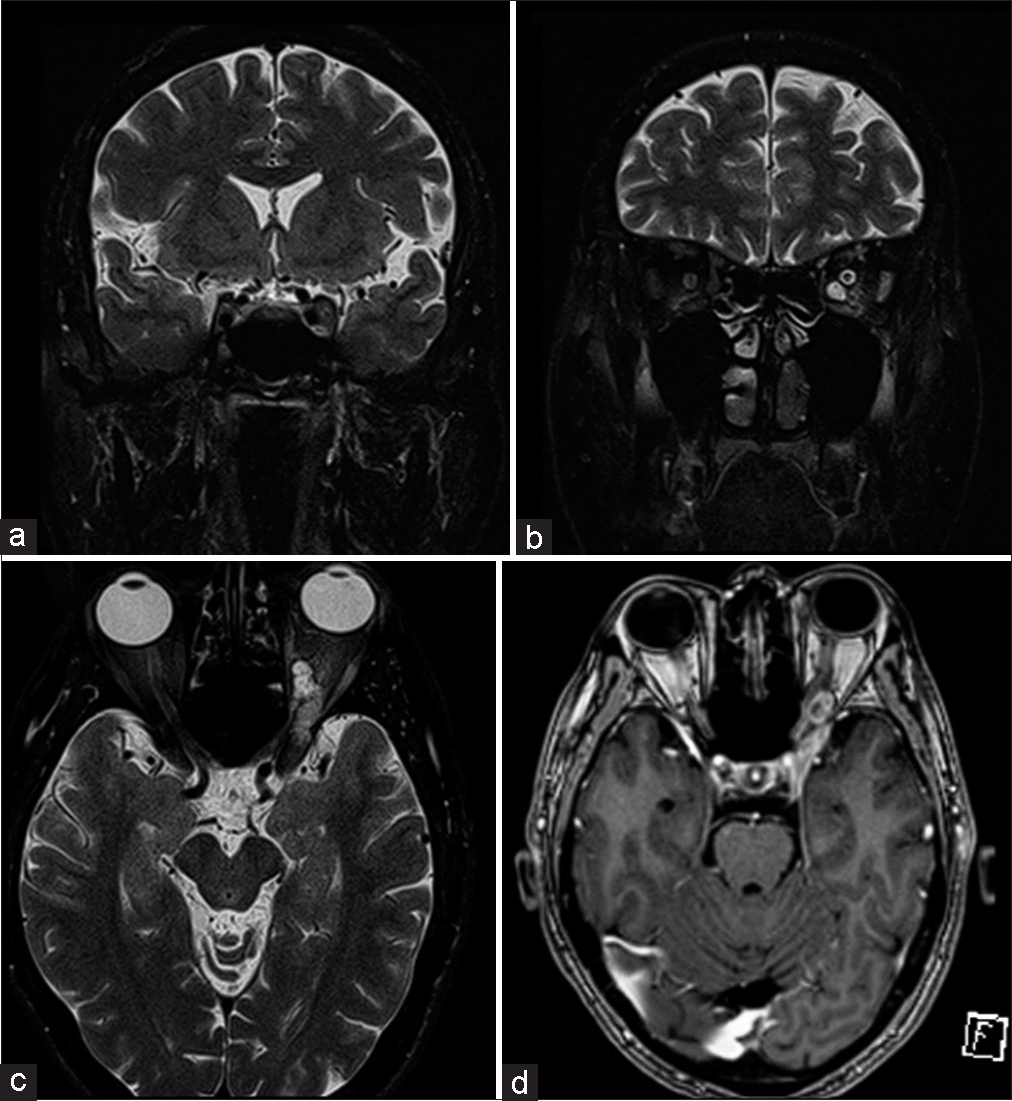
- Magnetic resonance imaging of the brain revealed (a) a 4.3 cm lesion occupying the superior portion of the left cavernous sinus and (b and c) extending into the left intraconal space. (d) It displayed heterogeneous enhancement on contrast imaging.
A lumbar subarachnoid drain (LSAD) was placed after induction of anesthesia, collecting 30 mL of cerebrospinal fluid (CSF) to facilitate brain relaxation and thereby ease interdural work. We approached the tumor through a left pterional craniotomy, orbitozygomatic osteotomy, and interdural approach, aimed at surgical excision and obtaining tissue for histopathology. We drilled the greater and lesser wings of the sphenoid bone and exposed the superior orbital fissure, foramen rotundum and foramen ovale. The middle meningeal artery was cauterized and cut. The orbitomeningeal band was cut and the outer dural layer was peeled off the lateral wall of the left cavernous sinus. Under direct visualization of the tumor in the lateral wall of the cavernous sinus, we performed the majority of our dissection between the V1 and V2 divisions, partially excising the lesion. Intraoperative examination of the frozen section of the lesion suggested a spindle cell tumor. We ensured hemostasis and closed the wound in layers. The LSAD remained in situ for the duration of surgery (3.5 h) and was removed immediately afterward.
He did not recruit any neurological deficits postoperatively. On the 3rd post-operative day (POD), we repeated an MRI to assess the extent of the residual tumor, noted in the intraconal space with a small component in the superior cavernous sinus. Incidentally, it revealed an extradural collection, 2.1 cm wide causing uncal herniation [Figure 2a and b]. We immediately re-explored the craniotomy, finding no definite collection, though the previously placed gel-foam sheet was soaked with CSF. A defect in the temporal dura was repaired, with fascia overlying the temporalis muscle. The dura was noted to have sunken inside and was therefore not opened.
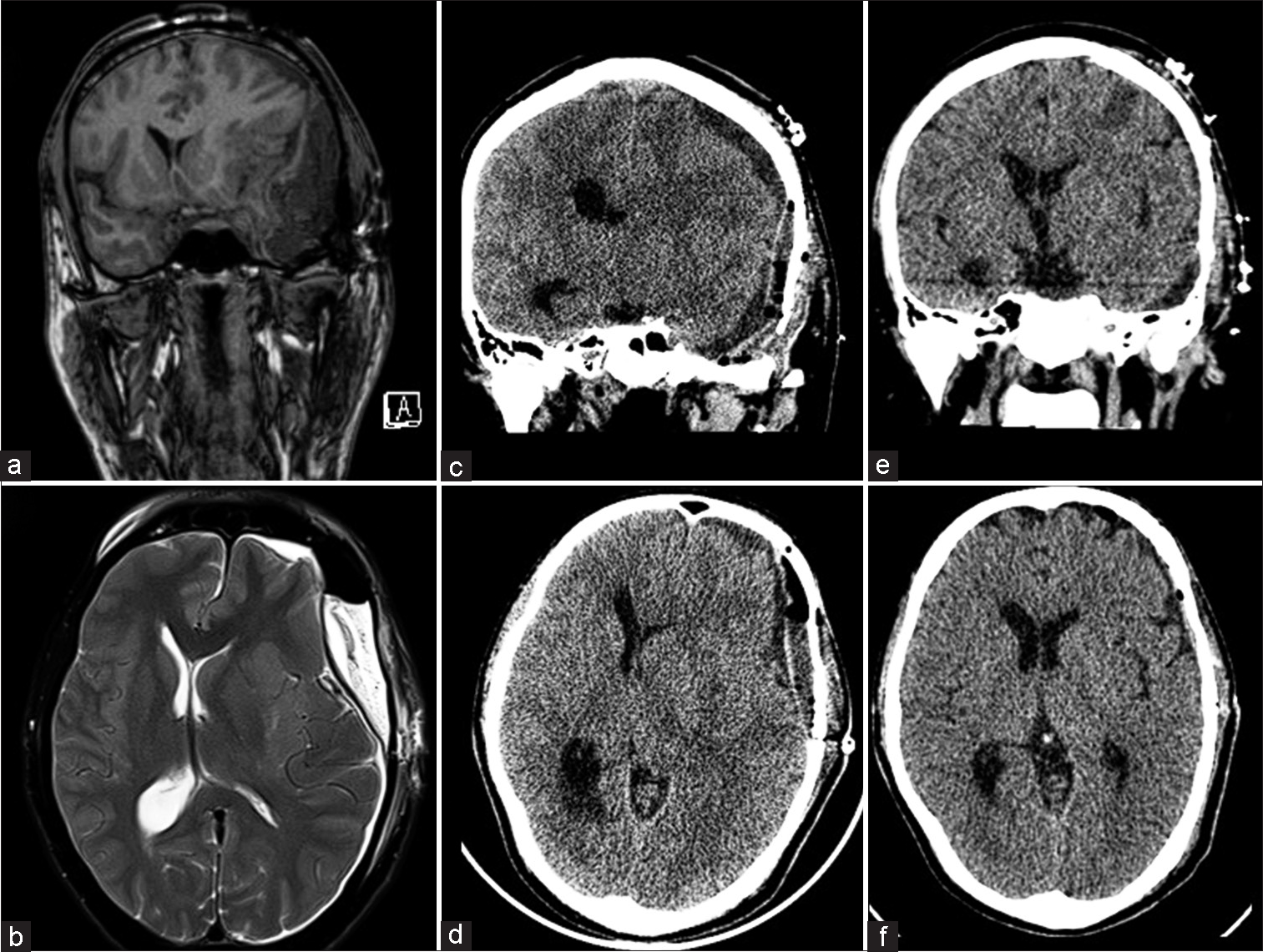
- (a) A post-operative day 3 magnetic resonance imaging brain revealed a large T1 hypointense, (b) T2 hyperintense extradural collection, 2.1 cm in width. Following evacuation of the collection, he did not develop any new symptoms; (c and d) however, a post-operative computed tomography revealed recollection in the subdural space, 1.3 cm thick, shifting the midline and nearly obliterating the left lateral ventricular system which was managed with simultaneous burr-hole evacuation of the collection and an epidural blood patch, (e and f) following which there was no recollection.
The patient recovered well, but despite his lack of symptoms, computed tomography (CT) performed on the 4th POD revealed a subdural collection with mass effect [Figure 2c and d].
We then postulated that the recurrent collections were secondary to intracranial hypotension (IH), the probable site of CSF egress being a dural puncture at the insertion of the LSAD. The subdural collection (comprised largely of CSF) was evacuated through frontal and parietal burr holes, followed by an epidural blood patch (EBP), injecting 30 mL of the patients blood in three bouts at the L4–5 level (the level of LSAD insertion) through an epidural catheter before extubation, to prevent further CSF leakage. Following this surgery, he remained asymptomatic, with no new neurological deficits. A CT scan 6 days later demonstrated evacuation of the subdural collection, with a resolution of mass effect [Figure 2e and f]. At the 13-month follow-up, he remained symptom-free.
DISCUSSION
IH is a clinical syndrome resulting from low CSF pressure; Spontaneous and iatrogenic IH are common,[1,2] with classical symptoms of orthostatic headache, worsening with coughing, nausea, vomiting, dizziness, and tinnitus.[3] A serious complication of IH is the rupture of bridging veins and sagging of the brain, leading to the occurrence of chronic bilateral subdural hematomas (SDH).
In our report, we highlight that the intracranial collections were unilateral, unlike prior reports of bilateral intracranial collections due to IH.[4] Their recalcitrant nature and lack of other precipitating factors made it clear that they were secondary to IH. Following the repair of the dural defect during initial re-exploration, communication between the subdural and extradural space was abolished, leading to recollection in the subdural space. IH has been described following diagnostic lumbar punctures;[5] a similar course likely occurred following the insertion of a LSAD in our patient. The striking lack of symptoms is explained by the fact that the collections were noticed on routine post-operative imaging rather than when they inexorably led to clinical deterioration.
Regardless of the cause of IH, EBP has been shown to be effective in relieving symptoms.[6] Alternate modes of treatment include steroids, hydration, caffeine, and bed rest. While an EBP closes the potential pathway for CSF leakage, the presence of an intracranial collection must be addressed. Indeed, thin, previously asymptomatic subdural collections due to IH may later cause mass effect after an EBP prevents egress of CSF and restores intracranial volume, requiring emergent evacuation.[7]
We previously employed this strategy in a case of spontaneous IH secondary to a dural diverticulum, evacuating bilateral SDH followed by an EBP leading to complete resolution, as in the present case.[8]
CONCLUSION
This report reiterates that IH should be deemed a possibility when dealing with unusual intracranial collections in the setting of any procedure that may risk compromising the integrity of the dura. Early recognition is imperative, and close monitoring with a low threshold for post-operative imaging is warranted in cases where subarachnoid catheters are placed. Evacuation of the collection while subsequently performing an EBP to prevent a recollection has been shown to be safe and effective in managing such cases.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- Management of iatrogenic spinal cerebrospinal fluid leaks: A cohort of 124 patients. Clin Neurol Neurosurg. 2018;170:61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome: A systematic review and meta-analysis. JAMA Neurol. 2021;78:329-37.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic imaging and clinical features of intracranial hypotension-review of literature. Pol J Radiol. 2017;82:842-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bilateral subdural hematomas caused by spontaneous intracranial hypotension. J Chin Med Assoc. 2008;71:147-51.
- [CrossRef] [PubMed] [Google Scholar]
- Life-threatening intracranial hypotension after diagnostic lumbar puncture. Neurol Sci. 2001;22:385-9.
- [CrossRef] [PubMed] [Google Scholar]
- Epidural blood patch for spontaneous intracranial hypotension with chronic subdural haematoma: A case report and literature review. J Int Med Res. 2016;44:976-81.
- [CrossRef] [PubMed] [Google Scholar]
- Value of targeted epidural blood patch and management of subdural hematoma in spontaneous intracranial hypotension: Case report and review of the literature. World Neurosurg. 2017;97:27-38.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous surgical decompression of bilateral subdural hematoma and an administration of epidural blood patch for spontaneous intracranial hypotension. J Neurosurg Anesthesiol. 2018;30:376-9.
- [CrossRef] [PubMed] [Google Scholar]






