Translate this page into:
Disseminated neurocysticercosis presenting as isolated acute monocular painless vision loss
Address for correspondence: Dr. Gaurav Mansukhlal Kasundra, 122, Subhash Nagar, Pal Road, Jodhpur - 342 008, Rajasthan, India. E-mail: gauravkasundra@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Neurocysticercosis, the most common parasitic infection of the nervous system, is known to affect the brain, eyes, muscular tissues and subcutaneous tissues. However, it is very rare for patients with ocular cysts to have concomitant cerebral cysts. Also, the dominant clinical manifestation of patients with cerebral cysts is either seizures or headache. We report a patient who presented with acute monocular painless vision loss due to intraocular submacular cysticercosis, who on investigation had multiple cerebral parenchymal cysticercal cysts, but never had any seizures. Although such a vision loss after initiation of antiparasitic treatment has been mentioned previously, acute monocular vision loss as the presenting feature of ocular cysticercosis is rare. We present a brief review of literature along with this case report.
Keywords
Acute permanent monocular vision loss
cysticercosis
intraocular neurocysticercosis
neurocysticercosis
subretinal neurocysticercosis
sudden monocular vision loss
Taenea solium
Introduction
Neurocysticercosis is not only the most common parasitic disease in developing countries, but all over the world.[1] It is caused by the larval stage of the tapeworm Taenea solium. Patients present with varied manifestations depending on the location and stage of the parasites and the degree of parasitemia.[12] We report a rare patient with combined ocular and cerebral neurocysticercosis who presented with acute painless monocular blindness prior to treatment with any antihelminthic drugs (AHD).
Case Report
A 41-year-old chronic alcoholic and smoker male patient residing in western India presented with history of acute painless monocular vision loss in right eye over seconds while drinking at home. The symptoms were non-progressive since onset, and the patient sought consultation after 4 days due to lack of any improvement in symptoms. The patient was diagnosed as being hypertensive since 5 years, but was not compliant with his medications and reported bilateral parieto-occipital mild heaviness-type of evening headache since 5 years without any other symptom. There was no early morning worsening of his headache or any associated complaints of vomiting or visual blurring over the last few years. There was no previous history of any focal neurological deficits, transient ischemic attacks, amaurosis fugax, ischemic heart disease or diabetes, or similar history in family members. General examination did not reveal any subcutaneous nodules or any other positive finding. Both pupils were of equal size and normally reacting to light and accommodation. He was able to count fingers at 1 m by the right eye, and acuity was 6/12 in the left eye. Color vision was normal in the left eye and was decreased appropriate to loss of acuity in the right eye. Fundus examination revealed focal anterior displacement of retina in right macula suggestive of a cystic lesion. Left eye fundus examination was normal and did not reveal any evidence of papilledema. Subsequently, full thickness retinal mapping was done which was suggestive of subretinal choroidal neurocysticercosis with greatly diminished visual field and retinal pigment epithelium/choroid disruption [Figure 1]. A brain magnetic resonance imaging (MRI) was done which revealed multiple intracerebral neurocysticercosis in both hemispheres without any significant mass effect or hydrocephalus [Figures 2 and 3] while MRI of the entire spinal cord was normal. The patient was advised surgical evacuation of the cyst followed by albendazole regime, but refused for surgery. AHD use in patients with ocular cysticercosis is associated with inflammatory response and retinal detachment leading to vision loss. He was thus started on treatment with only oral steroids given at 1 mg/kg dose and tapered off over 4 weeks. His visual acuity was static at 16 weeks of follow-up and is still not willing for surgical intervention. The patient does not report of any seizures till date and his electroencephalogram is repeatedly normal.

- (a) Full retina thickness map showing disruption of the right (OD) retina-choroid layers, and normal finding on left side (OS). (b) Global RPE/Choroid disruption map showing significantly impaired right side and normal left side
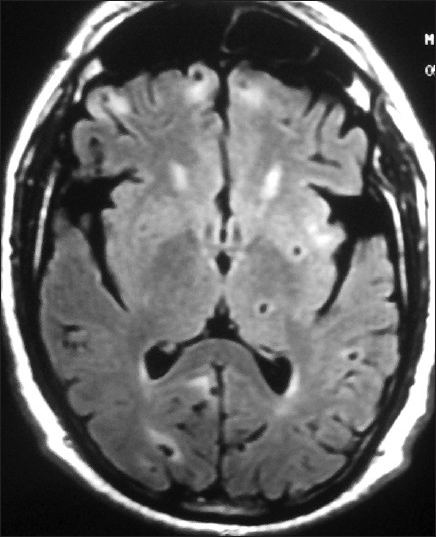
- Magnetic resonance imaging (MRI) brain axial view fluid attenuated inversion recovery (FLAIR) images showing multiple cysts of neurocysticercosis in varying stages of development
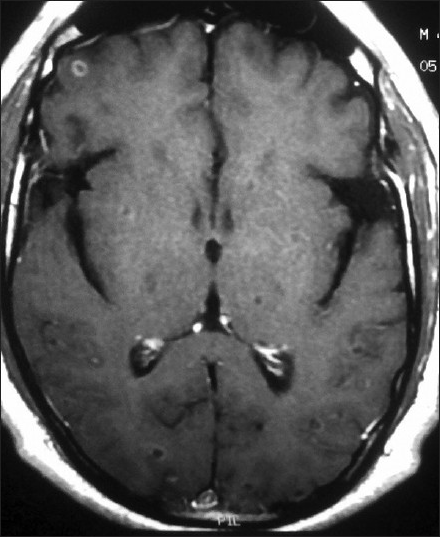
- MRI brain axial view post-gadolinium contrast images showing multiple cysts of neurocysticercosis in varying stages of development
Discussion
Cysticercosis was previously seen primarily in developing countries with poor hygiene, sanitation and slaughterhouse facilities.[34] It is now also seen in many western countries due to immigration from endemic areas and travel of local population to endemic areas. Man is the definitive host and pigs are the intermediate host. Human feces contain proglottids of the tapeworm, which contain the eggs within them. Humans may accidentally become intermediate hosts by ingesting these eggs via feco-oral contamination and subsequent hematogeneous spread of the cysticercii leads to their dissemination in the body.[4] Brain, eyes, muscles and subcutaneous tissue are most commonly involved, and accordingly the symptoms vary. The cysts reach the above organs including brain via a hematogeneous route. Similarly, the cysts reach the highly vascular choroid via the hematogeneous route and there it grows in size in the subretinal space. Ultimately it enters the vitreous to become an intra-vitreous cyst. Overall, the most common presenting symptom of cysticercosis is seizures (79%), followed by headache (38%), focal deficits (16%) and signs of raised intracranial tension (12%).[3] Visual symptoms are present in approximately 6% of the patients.[3] Cerebral involvement may be either parenchymal or extra-parenchymal, with the former presenting most commonly as seizures. Involvement of the eye may be as orbital or ocular. Within the orbit, the cysticercii involve the extraocular muscles and soft tissues.[5] Ocular cysticercii are intravitreal, subretinal, within the anterior chamber, or subconjunctival. Among patients with orbital and ocular cysticercosis, most common presentation is proptosis, followed by subconjunctival cyst, subretinal cyst, papilledema, atypical optic neuritis, lid nodule and lastly intraretinal cyst. Vision loss may be either due to intraocular cysts, due to chiasmal lesions causing optic nerve compression, due to retrochiasmal lesions like large parenchymal cysts or vasculitic cerebral infarcts, or due to hydrocephalus.[67] The mechanisms of vision loss, treatment and prognosis for visual recovery vary accordingly. The standard of care for cerebral neurocysticercosis is AHD (albendazole or praziquantel) with steroids and anticonvulsants. However, intraocular cysts if any should be removed prior to starting AHD, or else the tissue reaction to the dying parasite may lead to vitritis, retinal detachment and scarring leading to irreversible blindness.[89] Extraocular muscle cysticercosis also responds very well to AHD. In cases of isolated intraocular cysticercosis, timely surgical removal has been the treatment since the past 35 years or more.[10]
It is rare (10%) for patients with ocular cysticercosis to have concomitant cerebral parenchymal cysts.[2] We report such a rare patient with both organs being simultaneously involved. Disseminated neurocysticercosis presenting for the first time with acute monocular painless vision loss without any symptoms or signs suggestive of raised intracranial tension is extremely rare. There have been reports of post-AHD treatment acute monocular blindness,[89] but we could not find any with such a presentation prior to treatment initiation in the literature till date. The vision loss in our patient was due to the retinal pigment epithelium/choroid disruption secondary to a submacular cysticercus. Thus, in spite of having all risk factors for a vascular event and history suggestive of central retinal artery occlusion, we found a cause not thought of previously. Thus, it is important to rule out ocular causes of vision loss, as also consider cysticercosis in all such cases in spite of no other history suggestive of the disease. Also, the absence of seizures in spite of multiple cerebral parenchymal cysticercii is also uncommon considering that the most common presenting feature of neurocysticercosis is seizures.[3]
Acknowledgement
Md. Yasin and Janardan, Department of Neurology, Dr. Sampurnanand Medical College and Mahatma Gandhi Hospital, Jodhpur.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Pleomorphism of the clinical manifestations of neurocysticercosis. Trans R Soc Trop Med Hyg. 2006;100:134-41.
- [Google Scholar]
- Clinical manifestations associated with neurocysticercosis: A systematic review. PLoS Negl Trop Dis. 2011;5:e1152.
- [Google Scholar]
- Unusual presentation of orbital cysticercosis-ptosis, diminution of vision and medial rectus weakness: A case report. Cases J. 2009;2:7025.
- [Google Scholar]
- Neurocysticercosis presenting as hydrocephalus and bilateral optic atrophy. J Pediatr Neurosci. 2010;5:90-1.
- [Google Scholar]
- Monocular blindness during therapy for cerebral neurocysticercosis. J Assoc Physicians India. 2010;58:570-2.
- [Google Scholar]
- Rapid onset unilateral vision loss by intraocular cysticercosis demonstrated by MRI. Indian J Radiol Imaging. 1999;9:151-2.
- [Google Scholar]






