Translate this page into:
Reoperation in Spinal Dysraphism: Does it Help in Reversing the Neurological Deficits?
Address for correspondence: Dr. Praful Suresh Maste, Department of Neurosurgery, J N Medical College, KLES Prabhakar Kore Hospital and MRC, Nehru Nagar, Belagavi - 590 010, Karnataka, India. E-mail: drprafulmaste@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims:
After initial primary repair by inexperienced hands for the spectrum of pathological conditions in spinal dysraphism (SD), a few percentage of patients present with recurrent symptoms and worsening neurological status especially when primarily pathology is not identified and dealt properly. When the primary intradural tethering element is left untouched, worsening of symptoms is common. In this retrospective study, we tried to analyze the symptomatology, functional outcome at 1–2 months after the second surgery and associated complications.
Subjects and Methods:
All patients underwent second surgery at author's institution. Pre and post-operative data were evaluated using Necker –Enfants Malades (NEM) neurological and modified Hoffer ambulatory scale.
Results:
The main presenting complaints were bladder incontinence and limb weakness. Preoperative mean scores for motor and bladder were 3.56 and 2.78 out of 5, 2.67 out of 4, and 2.11 out of 3 for bowel and sensory function, respectively. Postoperative mean score for motor, sensory, bladder, and bowel function revealed good neurological improvement. Statistically neurological improvement in bladder and bowel function was significant. More than 60% of patients had normal ambulation at follow-up.
Conclusions:
Patients presenting with recurrent symptoms in an operated case of SD need to be investigated, cause of recurrence has to be identified, and if needed repeat surgery is recommended at the earliest. Long-standing neurological deficits can potentially improve, especially bladder and bowel function which gives a good quality of life to the patients. Furthermore, we want to stress the fact that since it is an intradural pathology, these cases should be operated by experienced neurosurgeons, and this fact should be made aware among referring doctors.
Keywords
Reoperation
spinal dysraphism
tethered cord
INTRODUCTION
After initial surgery in spinal dysraphism (SD), patient can again present with neurological deterioration which is often termed as recurrent tethered cord (RTC) syndrome. RTC can be due to untouched intradural pathology, inadequate detethering or retethering due to a variety of factors such as adhesions, large residual placode, small size of the canal, and nonrelease of the filum. The recurrence rate of 50% after 5 years of initially release and rate increases up to 57% by 2 years after the second release.[1] RTC usually follows after improper first surgery when local intradural pathology is not addressed and is common in patients who are operated by surgeons not trained in handling of neural tissue as well as improper technique. Literature from previous studies states patients presenting with RTC can improve after the second surgery and can lead a better functional life, even in long-standing neglected RTC cases. The main objectives of the study were to evaluate clinical symptoms and signs, functional outcome after second surgery, postoperative complications as well as highlighting the anatomical and technical aspects of tethered cord surgery.
SUBJECTS AND METHODS
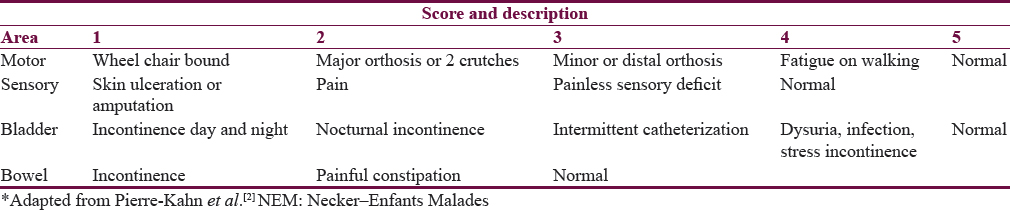
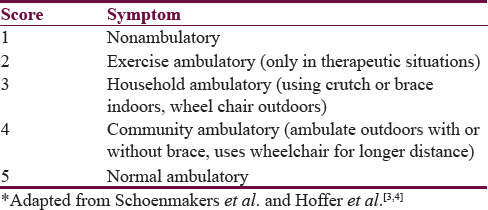
A retrospective analysis of patients presenting with symptoms and signs of RTC to the Department of Neurosurgery, J N Medical College and Dr. Prabhakar Kore Hospital and MRC, Belagavi, from January 2008 to December 2015, was done. The study included patients who were previously operated for SD and presented with features of RTC. A total of nine patients were included in the study. Patient's clinical features and indication for the first surgery was noted. All patients underwent magnetic resonance imaging (MRI) of spine and brain to evaluate the cause of RTC and rule out any other associated pathology such as hydrocephalus and Arnold-Chiari malformation. Patient's pre- and post-operative neurological status were assessed used (NEM) Necker –Enfants Malades neurological scale[2] [Table 1] and postoperative ambulation using modified Hoffer ambulatory scale [Table 2].[34] The test used for this study was independent sample t-test. The decision to perform surgery was based on clinical symptoms such as pain, lower limb weakness, sensory disturbances, bladder and bowel disturbance, neurological deterioration, and radiographic findings including low lying conus, tethering of the spinal cord to the subcutaneous scar or to theinner surface of the spinal canal and presence of intradural lipoma etc. All postoperative outcomes were assessed between 1 and 2 months.
RESULTS
In our series, the youngest patient was aged 1½ years, and oldest was 16 years old. Out of nine patients, six were male and three were female patients. The pathology included patients who were previously operated for meningomyelocele (MMC), lipomeningocele (LMM), tethered cord syndrome (TCS) dermal sinus tract with TCS [Table 3]. All of them had persistence of previous symptoms which were not resolved after first surgery associated with subsequent neurological deterioration.

Our patients presented with multiple complaints. However, the main presenting complaint was bladder incontinence (eight patients) followed by bowel incontinence, lower limb weakness, trophic ulcers, and foot deformities and backache [Figure 1].

- Clinical features in nine consecutive children operated previously
Age at first surgery and diagnosis, age at second surgery, and presenting complaints are (detailed) in Table 3. Preoperative neurological status of patients is shown in Table 4. The surgical procedure was optimized, individualized according to the etiology and specific pathology. The second surgery was aimed at dealing with the intradural pathology with detethering of cord and dysraphic elements with compulsory release of filum. Majority of cases were first operated by pediatric surgeons or in untrained hands who were not experts in dealing with pathology and possibly without the use of operating microscope. Imaging findings and operative procedure are detailed in Table 5.


Following surgery patients were assessed for neurological improvement [Table 6]. At follow-up of 8–10 weeks, all patients had clinical improvement and significant neurological improvement with six patients being ambulatory (score of 5) on modified Hoffer ambulatory scale [Table 6]. One patient had cerebrospinal fluid leak which needed re-exploration and repair while the second patient had wound infection which was treated with antibiotics [Table 7].


Analysis shows a operative mean score for motor, sensory, bladder, and bowel function was 3.56, 2.67, 2.78, and 2.11, respectively. Postoperative mean score for motor, sensory, bladder, and bowel function was 4.33, 3.56, 4.78, and 2.89, which indicates good neurological improvement [Table 8].
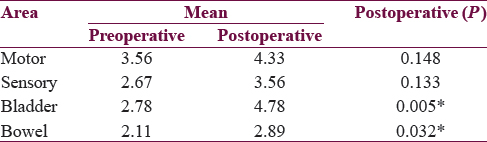
Statistically significant neurological improvement in bladder (P = 0.005) and bowel (P = 0.032) function was seen, but improvement in motor and sensory function was statistically insignificant because motor function and sensation was relatively less affected in comparison with bowel and bladder function at second presentation in our study. Follow-up imaging was not done in our study as the patient had no neurological deterioration during their follow-up, but imaging will help in monitoring patient's neurological status on a long-term basis. The limitation of our study was intraoperative neurophysiological monitoring, preoperative urodynamics, and small sample size but IONM and urodynamics evaluation may not be possible in very young children (<3yrs).
DISCUSSION
RTC is a controversial matter surrounded by debate in terms of management and if left untreated can lead to progressive neurological disability. Repair of MMC, LMM, etc., usually follows RTC resulting from the adhesions of the placode within a too narrow spinal canal. Ten percent of those with spinal lipoma and one-third of MMC develop symptomatic RTC, mainly caused by the ischemic-metabolic injuries due to the cord stretching and consequence of arachnoid scarring.[56789] Patients with RTC can present with sensorimotor, sphincter-related issues-bowel, and bladder problems which sometimes compels the child to quit schooling due to incontinence, social embarrassment, etc., and also there is a high risk of recurrent urinary tract infection secondary to incomplete emptying, hydronephrosis with renal involvement secondary to reflux. In addition, the patient can develop limb length discrepancy, orthopedics issues such as foot deformities and gait disturbances.
Phuong et al.[10] showed that majority of patients require end organ treatments who do not undergo untethering. Without surgery, patients will often experience a progressive neurological decline.[111213] It is important to know symptom characteristics, natural course of disease, and postoperative events to prevent complication and recurrence. Surgery is mandatory to prevent neurological worsening of symptoms. On a technical note, a good neuroanatomical knowledge, preoperative imaging, and renal workup along urodynamics are mandatory. The goal of surgical intervention is to deal with local pathology, debulking and disconnect the fibrous tissue, reconstruction of neural placode with aims to release the conus from the abnormal filum terminale as low as possible. Exposure should be good with caudal and cranial laminectomy; midline durotomy to expose the neural elements below the conus medullaris must be done without any traction on cord. Meticulous dissection using an operative microscope is mandatory due to the presence of extensive arachnoidal adhesion to ensure complete release of the spinal cord in a majority of the cases.
Following the duratomy, entire length of exposure should be checked for arachnoid bands, adhesion, and to identify rootlets. Filum can be identified with its typical dorsal midline location, slightly bluish color with its anteriorly located vessels, and the fat that often infiltrates it. The absence of filum anteriorly, one may have to search laterally and/or rostrally. Rootlets and arachnoid bands are difficult to differentiate at times. The rootlets at the sacral levels are directed to both sides and may be identified by their size and situation. The arachnoid bands attached to the dura are slightly transparent, flimsy and thinner in diameter when compared to rootlets. The use of IONM may be useful for safe surgery, but in the absence of this, good neuroanatomical knowledge is required to preserve the neural structures. Filum is sectioned after identification if not already sectioned during the previous operation which was the case in all of the patients in our series; it had not been cut during the previous operation. Detethering is confirmed intraoperatively by cranial ascent of filum. If not search for other cause such as bony spur, assessing the canal size, and the size of the remaining neural placode. Cutting placode dorsal to posterior rootlet line, over sewing of neural placode to accommodate it into the spinal canal with complete circumferential untethering of the spinal cord, finally cutting of ligamentum denticulatum and reconstruction of dural sac must be part of the surgery.
Herman et al.[14] reported on 153 patients with re-TCS (100 patients with MMC and 53 with spinal lipoma) who underwent untethering operations. These authors reported that motor complaints improved in 63% of patients, pain improved in 90% of patients, and bladder function improved in 35% of patients.[14] In thirty re-TCS surgeries, postoperative improvement was noted most often for pain (81%), and less often for urinary symptoms (53%), and weakness (48%).[15] The preoperative use urodynamics and Intraoperative Neuro Monitoring (IONM) need a mention here. The use of IONM is feasible in all TCS patients. The identification of functional nervous structures and continuous guarding of the integrity of sacral motor roots by IONM may contribute to the safety of surgical detethering.[16] Intraoperative use of microscope release of adhesion, detethering, identification of filum, and its release and water-tight closure of dural closure with layered wound closure are the prerequisites for a favorable postoperative outcome.
CONCLUSIONS
Retethering of spinal cord is a known phenomenon after primary surgery for tethered cord release caused due to various conditions like MMC, LMM etc. Interpretation of MRI is of paramount importance which is missed sometimes leading to non-recognition of pathology in the course of disease. Good release of tethered cord at first instance prevents recurrence in many patients and it has to be done by trained and experienced personnel, who have trained in this field. The challenge lies not only in the release of tethered cord but also in identifying patients with RTC. Second surgery has good outcome in the majority of patients especially in those with early signs and symptoms of retethering and also requires mandatory follow-up after second surgery with multi-disciplinary team. Awareness should be made among referral population, general practitioners, pediatricians, and obstetricians that treatment of RTC should be carried out by trained neurosurgeons, especially in developing countries where there are no specific referral guidelines. Finally to conclude, “an experience in handling such cases would give better results” and requires long term followup, rehabilitation and importance of at least an annual checkup for preventing and managing kidney damage in all patients of tethered cord release.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Surgical release of tethered spinal cord: Survivorship analysis and orthopedic outcome. J Pediatr Orthop. 1997;17:773-6.
- [Google Scholar]
- Long-term outcome of neurosurgical untethering on neurosegmental motor and ambulation levels. Dev Med Child Neurol. 2003;45:551-5.
- [Google Scholar]
- Functional ambulation in patients with myelomeningocele. J Bone Joint Surg Am. 1973;55:137-48.
- [Google Scholar]
- Recurrent tethered cord: Radiological investigation and management. Childs Nerv Syst. 2013;29:1601-9.
- [Google Scholar]
- Management and long-term follow-up review of children with lipomyelomeningocele, 1952-1987. J Neurosurg. 1990;73:48-52.
- [Google Scholar]
- Surgical treatment of the retethered spinal cord after repair of lipomyelomeningocele. J Neurosurg. 1991;74:709-14.
- [Google Scholar]
- Symptomatic retethering of the spinal cord after section of a tight filum terminale. Neurosurgery. 2011;68:1594-601.
- [Google Scholar]
- Natural history of tethered cord in patients with meningomyelocele. Neurosurgery. 2002;50:989-93.
- [Google Scholar]
- Diastematomyelia in 172 children: The impact of modern neuroradiology. Pediatr Neurosurg 1990. 1991;16:247-51.
- [Google Scholar]
- The tethered spinal cord: Diagnosis, significance, and management. Semin Pediatr Neurol. 1997;4:192-208.
- [Google Scholar]
- Role of surgery for maintaining urological function and prevention of retethering in the treatment of lipomeningomyelocele: Experience recorded in 75 lipomeningomyelocele patients. Childs Nerv Syst. 2003;19:23-9.
- [Google Scholar]
- Analysis of 153 patients with myelomeningocele or spinal lipoma reoperated upon for a tethered cord. Presentation, management and outcome. Pediatr Neurosurg. 1993;19:243-9.
- [Google Scholar]
- Outcome following multiple repeated spinal cord untethering operations. J Neurosurg. 2007;106(6 Suppl):434-8.
- [Google Scholar]
- The value of intraoperative neurophysiological monitoring in tethered cord surgery. Childs Nerv Syst. 2011;27:1445-52.
- [Google Scholar]






