Translate this page into:
Post hair transplant scalp arteriovenous fistula: A case illustration with a collective review
*Corresponding author: Arshad Ali, Department of Neurosurgery, Neuroscience Institute, Doha, Qatar. drarshadali@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ali A, Alrumaihi G, Al-Sulaiti G. Post hair transplant scalp arteriovenous fistula: A case illustration with a collective review. J Neurosci Rural Pract. doi: 10.25259/JNRP_451_2024
Abstract
Hair transplantation represents the most prevalent cosmetic procedure conducted among males globally; however, post-hair transplant scalp arteriovenous malformation (AVM) is an exceedingly rare complication. This report delineates a thorough review of all 18 previously documented cases identified through manual search and introduces a new illustrative case. A 31-year-old male exhibited progressively worsening headaches and a pulsatile scalp swelling over a span of seven years following the hair transplantation procedure. Diagnostic imaging-including magnetic resonance imaging, MR angiography, and digital subtraction angiography-confirmed the presence of a scalp fistulous AVM, primarily supplied by the left superficial temporal artery, along with additional contralateral feeders. A complete surgical resection was successfully executed in a hybrid operating room under intraoperative angiographic guidance. Although scalp AVMs post-hair transplantation are rare, they pose significant clinical risks; thus, prompt diagnosis and intervention, whether surgical or endovascular, are essential to prevent residual disease or recurrence.
Keywords
Arteriovenous malformation
Hair transplant complications
Intraoperative angiography
Post hair transplant
Scalp
INTRODUCTION
With over 1.2 billion people affected by male pattern baldness, hair transplantation is widely regarded as the leading cosmetic procedure among men, with more than 735,000 transplants performed globally each year.[1-3] Although hair transplant techniques and expertise have significantly advanced since their initial documentation over a century ago, reports of vascular complications related to these procedures remain rare.[4-8] Post hair transplant scalp arteriovenous malformation (AVM), often referred to as a cirsoid aneurysm, indicates the abnormal connection that develops between the arterial supply to the scalp and the draining veins, effectively bypassing the intermediate capillary bed.[9] If untreated for an extended period, scalp AVM can lead to severe neurological consequences, including acute intracranial hemorrhage, seizures, and significant neurological deficits, and may, in rare cases, result in death.[10-25] Following a thorough manual literature search, we have compiled 18 previously published reports on post hair transplant scalp AVM to date [Table 1]. This report aims to present our case while conducting a comprehensive review of all previously reported cases, with special attention to their clinical presentation, relationship with hair transplant techniques, neurodiagnostic features, and treatment options prognosis.
| No | First Author and year published | Age (yrs) |
Hair transplant technique |
Presentation/ Duration |
Location/predominent artery | Treatment option |
|---|---|---|---|---|---|---|
| 1 | Souder (1970) | 35 | PG | Headache. scalp bleeding/7 months | Lt temporal/ STA |
Surgical excision |
| 2 | Barros (1978) | 34 | PG | Ear throbing/ 9 months |
Rt temporal/ STA |
Surgical excision |
| 3 | Lanzieri (1985) | 64 | PG | Pulsating noise/ 3 weeks |
Rt temporal/ STA |
Surgical excision |
| 4 | Williams (1986) | 34 | PG | Pulsatile mass/ 2 yrs |
Lt Occipital/OA | Surgical excision |
| 5 | Semashko (1989) | 64 | PG | Pulsatile mass/ 3 weeks |
Rt temporal/ STA |
Ligation of STA |
| 6 | Semashko (1989) | 26 | PG | Pulsatile mass/ 1 month |
Rt temporal/ STA |
Ligation of STA |
| 7 | Fukuta (1994) | 44 | PG | Pulasatile mass/ 3 months |
Frontal/STrA and SOA | STA Ligation followed by Surgical excision |
| 8 | Tornambe (1994) | 35 | PG | Headache, pulsatile mass/4 months | Rt temporal/ STA |
Ligation of multiple feeders |
| 9 | Mathis (1994) | 27 | PG | Pulsatile mass/ 1 year |
NS/Bilateral STA and OA | Percutaneous (direct puncture) embolization |
| 10 | Davis (1997) | 45 | PG | Headache, pulsatile mass/15 years | Lt Occipital/ OA |
Endovascular embolization with surgical excision |
| 11 | Dogan (2008) | 27 | PG | Headache, pulsatile swelling/3-4 weeks | Rt temporal/STA | Endovascular embolization alone |
| 12 | Bernstein (2011) | 40 | NS | Enlarging mass/15 months | Lt temporal/ NS |
Patient refused angiography and treatment |
| 13 | Dabus (2014) | 30’s | NS | Enlarging mass and bruit/Several years | Lt fronto-temporal/STA | Percutaneous (direct puncture) embolization |
| 14 | Dabus (2014) | 40’s | NS | Enlarging mass and bruit/2 months | Rt occipital/ OA |
Endovascular embolization alone |
| 15 | Champeaux (2014) | 31 | NS | Enlarging mass and tinitus/2 years | Lt fronto-temporal/STA | Endovaascular embolization alone |
| 16 | Liounakos (2018) | 46 | NS | Headache and pulsatile mass/1 year | Lt parietal/STA and OA | Percutaneous (direct puncture) embolization |
| 17 | Molinaro (2022) |
29 | FUT | Headache & pulsatile mass/3 weeks | Rt parietal/ STA |
Endovascular embolization alone |
| 18 | Alfaro (2023) | 50 | NS | Enlaging painful mass/2 years | Rt Parieto-occipital/ STA and OA |
Percutaneous (direct puncture) embolization |
| 19 | Current Case | FUT | Headache and elarging pulsatile mass/7 years | Lt fronto-temporal/ STA bilaterally |
Surgical excision with intraoperartive angiography |
PG: Punch grafting, FUT: Follicular unit transplantation, STA: Superficial temporal artery, OA: Occipital artery, StrA: Supratrochlear artery, SOA: Supra-orbital artery, NS: Not specified, Lt: Left, Rt: Right.
CASE REPORT
A 31-year-old male initially presented 5 years ago with complaints of a throbbing headache and a pulsatile mass in the left frontotemporal region. Two years before this presentation, he underwent hair transplantation utilizing the follicular unit transplantation (FUT) technique. He reported swelling on the left side of his forehead just two weeks post hair transplantation, which progressively enlarged. His neurological examination was essentially unremarkable, while the local examination revealed a palpable bruit in the swollen scalp area, accompanied by prominent scalp vessels primarily on the left side of the head. He subsequently underwent magnetic resonance (MR) imaging (MRI) of the head with MR angiography [Figure 1]. This illustrated a left frontal scalp AVM characterized by multiple tortuous flow voids and dilated feeding and draining vessels. The left superior ophthalmic vein was also noted to be dilated. The primary feeding arteries originated from the dilated, tortuous left superficial temporal artery (STA), with drainage occurring through the dilated left facial vein. In addition, contralateral supply from the right external carotid artery and facial veins was observed. These findings correspond to a scalp AVM, also referred to as a cirsoid aneurysm of the scalp.

- (a and b) Magnetic resonance images (MRI) with contrast in axial and sagittal planes show a left frontotemporal vascular malformation in the scalp. (c) Magnetic resonance angiography (MRA) with 3D reconstruction displays the predominant left superficial temporal artery (green arrow), the fistulous point (yellow arrow), and the dilated vein drainage (white arrow).
His catheter cerebral angiography [Figure 2] showed a scalp AVM in the left anterior frontal area, predominantly supplied by the hypertrophied left STA, which appeared markedly tortuous and ended in a dilated venous pouch draining anteriorly into the left angular vein, both facial veins, and posteriorly into multiple superficial scalp veins. Numerous arterial feeders were identified, originating from the contralateral external carotid artery and the left ophthalmic artery. This was achieved through their anastomosis with the supraorbital and supratrochlear branches. The findings are consistent with a diagnosis of a scalp arteriovenous fistula (AVF) characterized by multiple feeding arteries bilaterally and superficial draining veins in the scalp, with no identified connections to the cerebral vasculature.
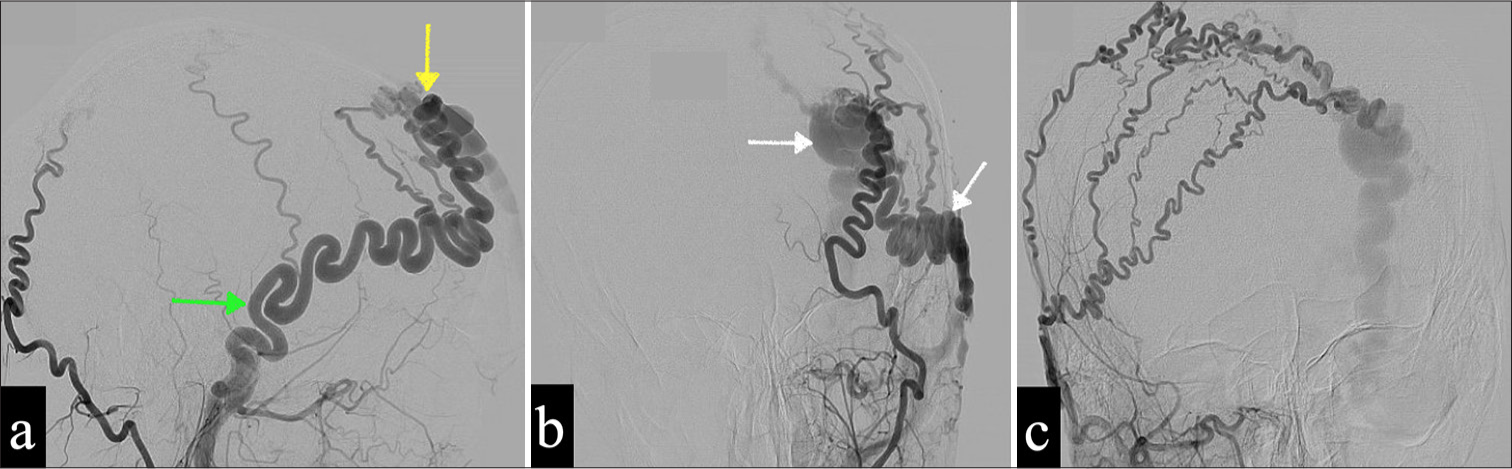
- (a and b) Pre-operative cerebral angiography of the selective left external carotid artery in the frontal and lateral planes shows predominant feeders from the left superficial temporal artery (STA) (green arrow), a fistulous point (yellow arrow), and dilated draining veins (white arrows). (c) There are supplementary feeding arteries contributed by the contralateral STA, which fill the draining veins.
The patient was offered various treatment options, including endovascular embolization and surgical excision. However, he declined all forms of treatment and was subsequently lost to follow-up. Upon his return visit, the patient exhibited a recurrence of worsening headaches, an increase in scalp swelling, and a single episode of recent epistaxis. He subsequently underwent repeat MRI, including MR and catheter cerebral angiography. The evaluation of the scalp AVM demonstrated a similar angioarchitecture, with the nidus increasing in size to 2.3 × 1.5 cm compared to the previous measurement of 1.7 × 1.4 cm recorded 5 years ago.
A multidisciplinary team (including neurosurgeons, neurointerventionists, plastic surgeons, and radiation oncologists) discussed the case in our fortnightly meeting regarding the treatment choice. They concluded that vascular malformation was not feasible for endovascular or percutaneous embolization due to its high-flow fistulous connections and tortuous angioarchitecture. A consensus decision was made to proceed with surgical excision with intraoperative cerebral angiography. The patient underwent surgery in a hybrid operating room (Brainsuite®, California, USA) equipped with an intraoperative 3D cerebral angiography suite (Artis Zeego®, Siemens Healthcare GmbH, Germany). After being positioned supine, the patient underwent cerebral angiography, and the fistulous point was localized using a laser grid integrated into the angiography gantry. Following the surgical excision, a femoral angiography sheath was left in situ for subsequent intraoperative angiography. A 6 cm curvilinear incision was made just posterior to the localized fistula in the coronal plane. Abnormally dilated, easily identifiable vessels were progressively coagulated as the skin incision was enlarged.
The main dilated vein was carefully dissected; the AVF was skeletonized and exposed. Afferent and efferent vessels to the AVF were coagulated. Two straight titanium Sugita® aneurysm clips (Mizuho Medical Co. Ltd, Tokyo, Japan) were placed on the STA before disconnecting. Then, the AVF was dissected from the deep surface of the reflected flap, above the temporal muscle, between the epicranial aponeurosis (galea aponeurotica) and the subcutaneous fat. The AVF was removed entirely without needing to remove the overlying skin. Before closing the wound, a post-excision intraoperative cerebral angiography [Figure 3a and b] was performed to ensure the complete excision of the fistulous nidus. Subsequently, the surgical flap was replaced and sutured. Blood loss was minimal, estimated at <150 mL, due to progressive vessel dissection and meticulous coagulation. Histopathology of excised tissue confirmed the diagnosis of AVM.

- (a and b) Intraoperative cerebral angiography pre- and post-excision of the fistulous nidus in lateral views shows no perfused nidus or draining veins. (c and d) Post-operative gadolinium-enhanced magnetic resonance imaging of the head after 8 weeks shows no residual or recurrence of the scalp arteriovenous malformation.
Postoperatively, the patient recovered completely with no symptoms. At the 3-month follow-up, he had fully recovered, with a well-healed surgical wound, no scalp necrosis, and the complete disappearance of prominent blood vessels on the scalp. A follow-up MRI [Figure 3c and d] of the head and MR angiography confirmed complete surgical excision.
DISCUSSION
The origins of successful hair transplantation trace back to as early as 1804 when Baromio conducted animal experiments. However, Okuda first documented the autologous hair transplantation technique in humans during the 1930s.[26-28] Since then, innovative techniques have transformed esthetic outcomes and enhanced safety profiles, with an overall complication rate ranging from 3% to 5%.[2,3,6,8,29,30] Notably, prominent studies on complications associated with hair transplants have not reported a single case of vascular complications, including scalp AVMs.[4-8] With advancements and refinements in endovascular and microsurgical techniques, there is renewed interest in scalp fistulae, particularly those that develop after hair transplantation.[9,30]
Hair transplant techniques and risk for vascular injuries
In the 1950s, Orentreich published a series of studies focused on hair transplantation, popularizing the 4-mm punch graft (PG) technique, recognized as the simplest and most effective method for hair restoration.[29,30] PG hair transplantation is typically performed as an office procedure involving harvesting 4 mm cylinders of autologous, full-thickness skin from hairy donor sites and their subsequent transplantation to bald recipient areas using a skin biopsy punch.[29,30,31] During the latter half of the 20th century, this technique underwent further refinement, evolving into FUT, also known as strip harvesting FUT.3 Subsequently, contemporary techniques have progressed toward greater minimal invasiveness by employing follicular unit extraction (FUE), which entails the direct removal of individual follicular units from the donor scalp, followed by their insertion into the recipient area through a two-step technique.[1-3] FUE has supplanted strip harvesting FUT to become the predominant technique for donor harvesting, resulting in enhanced cosmetic outcomes at both donor and recipient sites. Direct hair implantation (DHI) represents the latest technical advancement, wherein individually harvested hair follicles, similar to FUE, are immediately inserted into the recipient site, thus creating a one-step procedure.[1,2] Among the 18 reported cases, in 13 cases, either PG or FUT (including the present case) has been utilized. Nonetheless, it remains unclear whether the most recent minimally invasive procedures (FUE and DHI) demonstrate a reduced potential for scalp vascular injuries.
Clinical presentation
The most prevalent presenting complaints associated with scalp AVF include a progressively enlarging pulsatile mass or swelling, throbbing headaches, pulsatile tinnitus, scalp bleeding, and, in rare instances, epistaxis if left untreated for an extended duration.[10-25] This condition may develop as early as a few weeks, as evidenced in our case, or it may take several years from the initial presentation.[9] The STA or occipital artery typically serves as the predominant feeder, depending on whether the fistula has developed in the donor or recipient area. In addition, supplementary collateral feeding arteries may be recruited both ipsilaterally and contralaterally, as observed in our case.
Initial imaging techniques include computed tomography (CT) scans and CT angiography to delineate the angioarchitecture and facilitate the localization of the fistulous communication.[16] Alternatively, MRI and MR angiography have proven equally informative for diagnosing this scalp vascular malformation.[16,23] Digital subtraction cerebral angiography remains the gold standard for providing a comprehensive understanding of the fistulous connection of the nidus, differentiating between dominant and supplementary feeders. In addition, patients may undergo endovascular or percutaneous embolization procedures during the same session.[21,24,25] In our case, we employed intraoperative cerebral angiography as an adjunct to the microsurgical procedure to accurately localize the fistulous nidus and ensure complete excision.
Treatment options
Surgical excision, carried out in four cases, is the most durable, time-honored, and effective method for removing fistulous malformations in the scalp compared to the ligation-only method of the feeding artery, which was utilized in three cases. As evidenced in case number seven, the latter technique can potentially recruit collateral channels from the surrounding vascular network, specifically the ethmoidal arteries. With notable advancements in endovascular techniques over the past four decades, embolization has increasingly been utilized, using either percutaneous methods (four cases), intravascular techniques (four cases), or a combination of both in conjunction with surgical excision (one case).[32] In our case, since the neurointerventional team deemed embolization techniques to be unfeasible due to the high-flow fistulous connection and the lack of suitable equipment (such as appropriately sized intravascular balloons for proximal flow control, adequate expertise, and percutaneous sclerosing agents), we decided to proceed with the surgical excision of the nidus, using intraoperative cerebral angiography within a hybrid operating suite to ensure complete excision.
Prognosis
Regardless of the treatment options provided, this comprehensive review indicates no recurrence or residual nidus has been reported that would necessitate further management. Furthermore, no postoperative skin necrosis from vascular insufficiency was associated with any treatment modalities.[10-25]
CONCLUSION
Post hair transplantation AVFs are extremely rare; however, they can lead to significant complications. Clinical manifestations may be delayed several years after hair transplantation, necessitating timely diagnosis and appropriate imaging if suspected. Surgical excision has traditionally been the primary treatment option. However, there is a gradual shift in paradigm toward endovascular and/or percutaneous embolization in select cases, demonstrating satisfactory prognoses regardless of the treatment method used. Therefore, it is advisable to incorporate the potential for developing this rare yet serious vascular complication in the counseling process for this most commonly performed aesthetic procedure for male hair restoration.
Ethical approval:
The Institutional Review Board has waived ethical approval for this study.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Changing trends in surgical hair restoration: Use of Google trends and the ISHRS practice census survey. J Cosmet Dermatol. 2020;19:2974-81.
- [CrossRef] [PubMed] [Google Scholar]
- Practice census results. 2020. International Society of Hair Restoration Surgery. Available from: https://ishrs.org/wp-content/uploads/2020/05/report-2020-ishrs-practice-census-05-22-20.pdf [Last accessed on 2024 Dec 10]
- [Google Scholar]
- Hair transplantation: Basic overview. J Am Acad Dermatol. 2021;85:803-14.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of past and present hair replacement techniques. Aesthetic improvement, effectiveness, postoperative pain, and complications. Arch Facial Plast Surg. 1999;1:266-71.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of hair transplant procedures-causes and management. Indian J Plastic Surg. 2021;54:477-82.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of hair restoration surgery: A retrospective analysis. Int J Trichology. 2014;6:168-72.
- [CrossRef] [PubMed] [Google Scholar]
- Complications in hair restoration surgery. Oral Maxillofac Surg Clin North Am. 2009;21:119-48, vii
- [CrossRef] [PubMed] [Google Scholar]
- Surgical complications in hair transplantation: A series of 533 procedures. Aesthet Surg J. 2009;29:72-6.
- [CrossRef] [PubMed] [Google Scholar]
- Scalp cirsoid aneurysms: Case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-107.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula secondary to hair transplantation. N Engl J Med. 1970;283:473-4.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula after hair transplantation. Br Med J. 1978;1:340-1.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula after hair transplantation. AJNR Am J Neuroradiol. 1985;6:111-2.
- [Google Scholar]
- Hair transplantation producing arteriovenous fistulization. Ann Vasc Surg. 1986;1:241-3.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula following punch-graft hair transplantation. J Dermatol Surg Oncol. 1989;15:754-5.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula formation after punch graft hair transplantation in the frontal region. Plast Reconstr Surg. 1994;93:587-9.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistulae following hair transplantation: Collective review and report of a case. Ann Plast Surg. 1994;33:214-5.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula of the scalp after hair transplantation treated by endovascular embolization. Ann Plast Surg. 1994;33:633-7.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula of the scalp secondary to punch autograft hair transplantation: Angioarchitecture, histopathology, and endovascular and surgical therapy. Plast Reconstr Surg. 1997;100:242-9.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular treatment of AVF after hair transplantation. Cardiovasc Intervent Radiol. 2008;31:128-30.
- [CrossRef] [PubMed] [Google Scholar]
- Arteriovenous fistula following hair transplantation. Dermatol Surg. 2011;37:873-5.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular treatment for traumatic scalp arteriovenous fistulas: Results with onyx embolization. J Neurointerv Surg. 2014;6:405-8.
- [CrossRef] [PubMed] [Google Scholar]
- Scalp arteriovenous fistula following hair transplantation. Clin Neuroradiol. 2014;24:285-8.
- [CrossRef] [PubMed] [Google Scholar]
- Scalp arteriovenous fistula following hair transplantation: A case report and review of the literature. Clin Med Rev Case Rep. 2018;5:200.
- [CrossRef] [Google Scholar]
- Endovascular treatment of scalp AVF following hair transplantation: Case report. SN Compr Clin Med. 2022;4:245.
- [CrossRef] [Google Scholar]
- Traumatic scalp arteriovenous fistula post capillary implantation successfully treated using PHIL embolic agent. Surg Neurol Int. 2023;14:12.
- [CrossRef] [PubMed] [Google Scholar]
- Cirsoid aneurism of the scalp: With the report of an advanced case. Ann Surg. 1924;80:332-40.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and experimental studies of transplantation of living hairs. Jpn J Dermatol Urol. 1939;46:537-87.
- [Google Scholar]
- Autografts in alopecias and other selected dermatological conditions. Ann NY Acad Sci. 1959;83:463-79.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical pearl: Punch biopsy technique for alopecias. Int J Womens Dermatol. 2022;8:e054.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular treatment of scalp cirsoid aneurysms. Radiol Bras. 2010;43:224-8.
- [CrossRef] [Google Scholar]







