Translate this page into:
Ictal Blinking in Hepatic Encephalopathy Pre- and Post-Liver Transplant: Report of Eight Patients
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective Seizures are reported in 20 to 30% of cases with chronic liver disease in association with hepatic encephalopathy. Majority of these are focal seizures. Ictal blinking is reported first time in these patients pre- and post-liver transplant.
Methods From November 2018 to October 2021, retrospective data was analyzed in patients with end-stage liver disease and hepatic encephalopathy, both pre- and post-liver transplant.
Results Eight patients had ictal blinking, four were pre-transplant and four post-transplant. Five patients (four after liver transplant and one pre-transplant) were seizure free, three died of liver disease and multiorgan dysfunction, and one did not follow-up.
Conclusion Ictal blinking in relation to liver disease and hepatic encephalopathy is reported, often missed and requires short duration antiepileptic medications.
Keywords
chronic liver disease
cirrhosis
hepatic encephalopathy
ictal blinking
EEG
MRI
CT
Introduction
Eye lid closure voluntary or involuntary is called blinking. This may be habitual as seen in tics which can be stopped at will. When the blinking is involuntary, it is a seizure phenomenon. It is difficult to differentiate ictal blinking (IB) from eyelid myoclonia (EM). EM with or without absences associated with polyspikes and waves discharges in EEG on eye closure, is an epileptic syndrome called Jeavon's syndrome, commonly seen in children.1 Isolated IB with or without eyes deviation, without spread to other parts of body and without polyspike wave discharges on eye closure, is very rare.2 IB is different from Jeavon's syndrome.1 2 There may be involvement of orbicularis oculi muscles in IB. No specific area of the brain is particularly involved and EEG discharges could arise from anywhere in brain.2 There are few reports of IB in adults and children.2 3 We report eight cases of IB in chronic liver disease (cirrhosis) with hepatic encephalopathy (HE). Frequent eye blinking is also seen in blepharospasm and photosensitive epilepsy. Both these are out of context in this case series as all the cases were seen in relation to the end stage liver disease in an intensive care unit setting.
Materials and Methods
Institute of liver and biliary sciences (ILBS), New Delhi is an institute of its kind in India exclusively for management of liver disease patients. On an average 100,000 patients are seen in outpatient clinic per year and about 10,000 patients are admitted in intensive care unit. At ILBS about 100 live and deceased liver transplantation (LDLT/DDLT) are done per year. Eight cases of IB/EM were seen from November 2018 to October 2021, in patients with cirrhosis, HE, and following liver transplantation. All the patients had end-stage liver disease. All four in liver intensive care unit were intubated and on propofol, rest four were in liver transplant ICU, extubated, and not on propofol. IB was diagnosed in these patients at the time of neurological examination. The neurological opinion was sought to find out the cause of deranged sensorium not explained on the liver failure or other systemic reasons. IB patients were investigated for the underlying cause (EEG, MRI brain, metabolic work-up). All the patients received intravenous midazolam 5 mg (except case 7) and levetiracetam 1G loading followed by 750 mg 12 hourly. There was one child (case 4) only and he received intravenous levetiracetam 40 mg/kg loading followed by 20 mg/kg/d in two doses per day. EEG recording was not done at the time of diagnosis in seven patients. There was time lag of 1 to 4 hours between noting IB and recording of EEG. Only one patient (case no 2) had an EEG before loading with intravenous midazolam and levetiracetam. Videos of the patients were recorded with smart phones. Imaging of the brain (MRI/CT) was done over next few days. MRI/CT were routine ones and not as per epilepsy protocol. Continuous EEG monitoring was not done in any patient. Two representative videos and three EEGs are described in this report. Oral levetiracetam was continued for 3 months after initial loading. Four patients with liver transplant were following up regularly. Follow-up ranged between 6 months to 33 months. All were seizure free and off levetiracetam. Only one patient had a repeat EEG before stopping levetiracetam. One patient with ethanol-related liver disease and IB was lost to follow-up after first admission. Three patients out of eight had passed away and did not undergo liver transplantation (Table 1).
|
S no |
Age/sex |
Diagnosis |
Seizure semiology |
EEG |
MRI/CT |
Treatment Mdz,Lev,Lac |
Outcome |
Transplant |
Other medications |
|---|---|---|---|---|---|---|---|---|---|
|
1 |
48/M |
Cirrhosis liver, cryptogenic |
Ictal blinking, orbicularis oculi, post-LT |
Normal |
CT volume loss |
Mdz., Lev |
Seizure free |
Deceased LT |
Methyl prednisolone, Basiliximab, tacrolimus |
|
2 |
56/M |
Cirrhosis Hep B/C |
Ictal blinking, post-LT |
Frontotemporal spikes, generalization |
MRI normal |
Mdz., Lev |
Seizure free |
Live donor LT |
Methyl prednisolone, tacrolimus |
|
3 |
42/M |
Cirrhosis, Ethanol related |
Ictal blinking |
Periodic spikes, burst suppression |
CT volume loss |
Mdz. Lev., Lac. |
Died of sepsis, DIC |
No |
No |
|
4 |
9y/M |
Acute liver failure, undetermined |
Ictal blinking with eyes deviation post-LT |
Normal |
MRI normal |
Mdz. Lev. |
Seizure free |
Live donor LT |
Methyl prednisolone, tacrolimus |
|
5 |
50/M |
Cirrhosis, Ethanol related |
Blinking |
Periodic spikes, focal discharges |
CT normal |
Mdz. Lev., Lac. |
Died of sepsis, DIC |
No |
No |
|
6 |
55/F |
Cirrhosis, scleroderma, disseminated tuberculosis |
Ictal blinking |
Focal seizure discharges |
CT normal |
Meds., Lev. |
Died of sepsis/disseminated tuberculosis |
No |
No |
|
7 |
52/M |
Cirrhosis, Ethanol related |
Ictal blinking and orbicularis oculi |
Generalized spike discharges |
MRI/CT Subdural hematoma |
Lev |
Seizure free |
No |
Propofol |
|
8 |
51/M |
Cirrhosis Cryptogenic |
Ictal blinking and right forearm jerks |
Generalized spike discharges |
MRI/CT normal |
Lev |
Seizure free |
Live donor LT |
None |
Abbreviations: CT, computerized tomography; DIC, disseminated intravenous coagulopathy; Lac., lacosamide; Lev., levetiracetam; LT, liver transplant; Mdz., midazolam; MRI, magnet resonance imaging.
Cases
Case 1
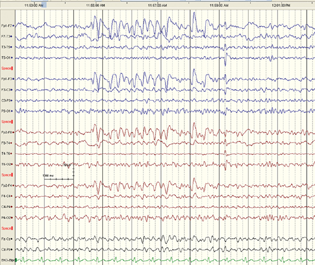
A 48-year-old male diagnosed with cirrhosis of unknown cause with portal hypertension, ascites, jaundice, and grade three esophageal varices, underwent dec eased liver transplant in January 2019. During transplant he was given methylprednisolone 500 mg, basiliximab 20 mg, and tacrolimus 2 mg. He was extubated on day two of transplant. On day five, following liver transplant he was noted to have some alteration of sensorium. Neurological examination revealed IB seizures involving even orbicularis oculi with some times head and eyes deviation to right (Video 1). He was not responding to oral commands. Each time the episode of blinking lasted for 1 to 3 minutes. Injection midazolam 5 mg was given followed by intravenous levetiracetam 1G. The eye blinking stopped within 1 minute of taking midazolam. EEG done after 3 hours of ictus showed bifrontal sharp waves, spike wave discharges (Fig. 1). Serum electrolytes like sodium, calcium, and magnesium were normal. Kidney functions were normal. Serum ammonia was 88 (31–123 ug/L). Serum tacrolimus level was 2.4 (10–20 ug/L). CT brain revealed decreased brain volume. Levetiracetam was continued for 3 months on follow-up and stopped after that. He had no seizures since then.
-
Fig. 1 Case 1: a 48-year old man with cirrhosis, portal hypertension, and hepatic encephalopathy with ictal blinking post-liver transplant, EEG shows bifrontal sharp discharges.
Fig. 1 Case 1: a 48-year old man with cirrhosis, portal hypertension, and hepatic encephalopathy with ictal blinking post-liver transplant, EEG shows bifrontal sharp discharges.
Case 2
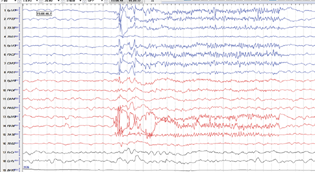
A 56-year-old male had hepatitis B and C-induced cirrhosis and underwent live–liver donor transplantation in August 2021. On day 25 post-liver transplant he was noted to be unresponsive and not communicating. Examination revealed frequent eye blinking and some eye deviations. EEG done at the same time revealed frequent frontotemporal spikes discharge lasting for 5 seconds at several places (Fig. 2). At times generalization was also seen. He was treated with 5-mg intravenous midazolam and levetiracetam 1G intravenously. This was followed by levetiracetam 750 mg twice a day. Serum electrolytes like calcium and magnesium were normal. Serum sodium was 150 (136–145 µmol/L). Kidney functions were normal. Serum ammonia was 72 (31–123 µg/L). Serum tacrolimus level was 6.9 (5–15 µg/L). His MRI brain was normal. Levetiracetam was stopped after 3 months.
-
Fig. 2 Case 2: a 56-year-old man with cirrhosis post-hepatitis B and C had IB on day 25 post-liver transplant. EEG shows frontotemporal spike discharges.
Fig. 2 Case 2: a 56-year-old man with cirrhosis post-hepatitis B and C had IB on day 25 post-liver transplant. EEG shows frontotemporal spike discharges.
Case 3
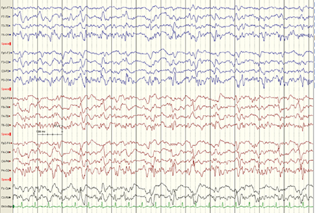
A 42-year-old male, suffering from ethanol-induced cirrhosis with HE, had liver failure with ascites, jaundice, coagulopathy, pneumonia, septic shock, and disseminated intravascular coagulopathy. He was admitted in December 2018. He was intubated and was on propofol, fentanyl, and atracurium. In the ICU he was noted to have frequent eye blinking. EEG revealed spike and wave discharges arising from temporo-occipital region (Fig. 3). Intravenous 5-mg midazolam followed by levetiracetam 1G controlled the seizures. Serum electrolytes like sodium, calcium, magnesium were normal. Kidney functions were normal. Serum ammonia was 270 (31–123 µg/L). A repeat EEG after 24 hours revealed no abnormal discharges. However his basic underlying disease worsened and last EEG had triphasic waves and burst suppression. The patient died after 2 days with multiorgan dysfunction as no donor was available.
-
Fig. 3 Case 3: a 42-year old man with ethanol-related cirrhosis, hepatic encephalopathy and shock, EEG shows temporo-occipital spike wave discharges, slightly more on left side.
Fig. 3 Case 3: a 42-year old man with ethanol-related cirrhosis, hepatic encephalopathy and shock, EEG shows temporo-occipital spike wave discharges, slightly more on left side.
Case 4
A 9-year 4-month-old boy with acute fulminant liver failure of unknown cause underwent emergency live–liver donor transplant in January 2019. He was extubated on day 2. After extubation the nurses noted the boy was frequently blinking and there was an eye deviation to right. He was not responding to commands. There was no associated convulsions of rest of the body. He was treated with intravenous midazolam and levetiracetam. EEG done after 3 hours of ictus was normal. MRI brain was normal. Serum electrolytes like sodium, calcium, and magnesium were normal. Kidney functions were normal. Serum ammonia was 112 (31–123 µg/L). Serum tacrolimus level was 4.7 (10–20 µg/L). He remained seizure free on follow-up and levetiracetam was discontinued after 3 months.
Case 5
A 50-year-old male with ethanol-related chronic liver disease with encephalopathy was admitted to the intensive care unit in March 2019. He required intubation on the day of admission. During his hospitalization he was noted to have frequent eye blinking. He was given one dose of midazolam 5 mg and 1G of levetiracetam. EEG revealed periodic spike discharges, triphasic waves. At the time of seizures. serum electrolytes like sodium, calcium, and magnesium were normal. Kidney functions were normal. Serum ammonia was 150 (31–123 µg/L). His condition worsened over time and had multiorgan failure. The patient passed away 3 days after this. There was no donor available for liver transplantation.
Case 6
A 55-year-old female had cirrhosis, scleroderma, and disseminated tuberculosis. She was intubated on propofol, fentanyl, and atracurium. During examination eye blinking was noted. EEG revealed focal seizure discharges. MRI brain was unremarkable, but PETCT revealed hot areas on left and right temporal regions. A differential diagnosis of herpetic encephalitis, vasculitis of brain was considered. She was in addition treated for herpes simplex encephalitis. Blood for herpes DNA was negative. A cerebrospinal fluid could not be performed in view of coagulopathy. Serum electrolytes like sodium, calcium, and magnesium were normal. She started to deteriorate and she had renal failure (urea was 298, creatinine 2.56). Serum ammonia was 83.6 (31–123 µg/L). She had multiorgan failure and passed away.
Case 7
A 52-year-old male with ethanol-related cirrhosis was noted to have eye blinking with orbicularis oculi while being weaned off propofol. Electroencephalography revealed occasional generalized spike discharges. Intravenous levetiracetam was given 1G followed by 750 mg twice a day. There was no recurrence. MRI brain and CT scan revealed 1 cm rim of subdural hematoma on the right side. There was no parenchymal lesion. His serum magnesium was normal. Calcium was borderline low 7.5(8.4–10.4 µmol/L) and sodium was 149 (136–145 µmol/L). Platelets were low 40,000/m3, ammonia was 83 (31–123 µg/dL). He was discharged after 1 week of hospitalization. He did not follow-up after the discharge from hospital.
Case 8
A 51-year-old male patient with cirrhosis underwent live–liver donor transplantation in October 2021. On day 45, he was found drowsy and not responding to commands. Previously he had hypernatremia of 160 mEq/L and hypocalcemia. Both these metabolic derangements were corrected a day before. His serum ammonia was normal and he was not on tacrolimus. Examination revealed frequent eye lid blinking and occasional focal jerks of the right upper limb (Video 2) There was no spread of these abnormal movements in any other body parts. He was not responsive. EEG revealed seizure discharges and he was given intravenous 1 G of levetiracetam later followed by 750 mg twice a day. These seizures were controlled with this treatment. His MRI brain was normal. Levetiracetam was stopped in January 2022.
Discussion
Seizures are reported in about 30% of the HE patients.4 5 Majority of these seizures are focal seizures.5 Generalized tonic–clonic seizures are uncommon and mostly are seen in pre-intubation and alcohol withdrawal patients with HE. After intubation the seizures are focal and nonconvulsive type. Propofol is the most common anesthetic agent used during ventilation. Propofol itself has an anticonvulsant effect and may be the reason that generalized tonic–clonic seizures are not seen in these patients.6 IB was noted in eight patients. In a previous study based on EEG in HE over a period of 6 months from ILBS, 30 cases of seizures were seen out of 151 patients. This gives a rough estimate of 180 seizure cases over 3 years. Out of these, eight cases had IB, making it 4.4% of all seizures.4 Is IB same as EM? Literally speaking they mean same. However, EM with or without absence associated with polyspikes and wave discharges in EEG on eye closure, is an epileptic syndrome, commonly seen in children.1 Isolated IB with or without eyes deviation, and without spread to other parts of body is very rare.2 There may be involvement of orbicularis oculi muscles in IB. No specific area of the brain is particularly involved and EEG discharges could arise from anywhere in brain.2 Four patients (three children) with IB have been reported in the literature.2 Excessive blinking is seen in dry eyes, tics, blepharospasm, Jevon's syndrome, and photosensitive seizures. Pure IB involves only eyelids without other facial muscles involvement or other body parts. This could be unilateral or bilateral. When it is unilateral blinking it is more specific lateralizing sign of partial seizures.7 8 9 Isolated or pure blinking has been described in two children.8 10 However, in one study of 61 adults with EM, the authors divided them into three groups based on history, seizure semiology, and EEG findings.3 Group 1 had 31 patients with typical EM with or without absences. Second group of 20 patients with EM had associated generalized tonic–clonic seizures. The third group of seven patients had symptomatic epilepsies. In their conclusion it was noted that EM could be seen as a seizure in symptomatic epilepsies.
Pathogenesis and etiology of IB are not clear.8 Blinking is a reflex mechanism involving trigeminal, oculomotor, and facial nerves. Anatomically blink reflex is a brainstem reflex and is produced on stimulation/irritation of the sensory nerves, motor nerves of the brain.11 Ipsilateral precentral cortex stimulation has produced the IB.12 Ipsilateral cerebellum, frontal lobe, basal regions of temporal lobes, occipital areas, and basal hemisphere structures could be involved in IB.2 8 10 12 In one study of 14 patients, a contralateral lesion of the cortex was found in ipsilateral blinking (mono-ocular blinking).7 Unilateral eye blinking was seen in 11 children with tuberous sclerosis but it had no localizing value.13 Eye blinking has been reported in association with cerebellar ganglioma.14 But EEG had seizure discharges arising from left frontopolar region. Intraoperative recording had electrical discharges arising from cerebellar and frontal cortex.14 Bilateral blinking was reported in a fourth ventricular mass.15 Two adults with unilateral and bilateral blinking have been reported in focal epilepsy.12 All these associations prove that IB had no localizing value. The most recent publication on IB reported unilateral eye blinking was more useful localizing sign as compared with bilateral blinking which had no localizing value.9 In unilateral blinking the localization was mostly in opposite frontotemporal region on EEG. In this series IB constituted 2.2% of focal seizures.9 In our patients IB was approximately 4.4% of all seizure cases. All eight patients except case 7 in this report had a routine 20 minutes EEG after 1 to 4 hours after noting IB. Six of eight patients had EEG abnormality and other two had no seizure discharges.. None of the patients underwent continuous EEG monitoring. Poor localizing value of EEG abnormality in IB has already been reported previously, and this was also seen in our series.2 7 8 9 10 12
Tacrolimus is used in liver transplant patients as immunosuppressant to guard against rejection of the transplanted liver.16 Tacrolimus has known neurotoxicity and known precipitant of seizures. However, only three patients of eight had exposure to tacrolimus. The patients were on tacrolimus from the day of receiving liver transplant to IB (day 2 to 25). Tacrolimus levels were in low therapeutic range in all the three. Hence a correlation between tacrolimus and IB is unlikely.
Eight patients with IB are described in this report, two of these had pure IB and rest had eyes deviations or spread to other face muscles. All our patients had end-stage liver disease and managed in ICU. All eight had HE without structural brain lesions. Brain imaging was normal in five, two had brain volume loss, and one had a thin rim of subdural hematoma. There was no previous history of seizures in these patients. EEG was abnormal in six. What triggered IB in these patients is not exactly clear. Hyperammonemia, brain edema, tacrolimus or other unknown metabolic factors could be the possible cause in them for IB. But at the time of IB, none of the above causes were seen. Possible after effect of hyper ammonia previously and other systemic factors in an encephalopathy state could be the reasons. From the available clinical information, limited investigations, focal EEG abnormalities in some, in this case series it may not be safe to recommend early discontinuation of the antiseizure medications. However, all were given antiepileptic drug for 3 months only. There was no recurrence of seizures on follow-up in four of them, post-transplant. One case did not follow-up and three passed away. The patients are following up regularly at this hospital till date. No post-mortem autopsy was performed.
Conclusion
IB/eye lid myoclonia in the setting of HE is not reported. IB/IM is a condition which was seen in HE patients before and after liver transplant. These seizures do not require long-term antiepileptic medications. If HE patient has unexplained obtundation/unresponsiveness, observe eyes for IB/EM. Continuous EEG monitoring is recommended in such patients as nonconvulsive seizures may be there as well. Mechanism is unknown, as all the patients had normal metabolic work-up and brain imaging at the time of IB/EM. All these patients had no previous history of seizures.
Limitations of the Study
All patients had routine MRI/CT and EEGs. Special MRIs with epilepsy protocol were not done. Continuous EEG monitoring was not done in any patient. Cerebrospinal fluid examination was not done in view of the coagulopathy associated with the underlying liver disease.
Conflict of Interest
None declared.
References
- Jeavons syndrome: clinical features and response to treatment. Pediatr Neurol. 2018;86:46-51.
- [Google Scholar]
- Ictal blinking, an under-recognized phenomenon: our experience and literature review. Neuropsychiatr Dis Treat. 2017;13:1435-1439.
- [Google Scholar]
- Eyelid myoclonia seizures in adults: an alternate look at the syndrome paradox. Epilepsy Behav. 2015;45:265-270.
- [Google Scholar]
- Role of EEG in predicting outcome of hepatic encephalopathy patients. Neurodiagn J. 2020;60(4):272-288.
- [Google Scholar]
- Management of agitation and convulsions in hepatic encephalopathy. Indian J Gastroenterol. 2003;22(02):S54-S58.
- [Google Scholar]
- The anticonvulsant effects of propofol and a propofol analog, 2,6-diisopropyl-4-(1-hydroxy-2,2,2-trifluoroethyl)phenol, in a 6 Hz partial seizure model. Anesth Analg. 2011;112(2):340-344.
- [Google Scholar]
- Unilateral blinking: a lateralizing sign in partial seizures. Neurology. 1996;46(1):45-48.
- [Google Scholar]
- Unilateral eye blinking arising from the ictal ipsilateral occipital area. Clin EEG Neurosci. 2016;47(3):243-246.
- [Google Scholar]
- Ictal blinking: reappraisal of the lateralization and localization value in focal seizures. Clin EEG Neurosci. 2022;5:15. 500594211070800
- [Google Scholar]
- Ipsilateral blinking seizures during left fronto-temporal ictal pattern on scalp EEG. Epileptic Disord. 2007;9(4):449-452.
- [Google Scholar]
- Brainstem reflexes: electrodiagnostic techniques, physiology, normative data, and clinical applications. Muscle Nerve. 2002;26(1):14-30.
- [Google Scholar]
- Ictal unilateral eye blinking and contralateral blink inhibition—a video-EEG study and review of the literature. Epilepsy Behav Case Rep. 2013;1:161-165.
- [Google Scholar]
- Ictal unilateral blinking is an unreliable lateralizing sign in tuberous sclerosis complex. Epilepsy Res. 2016;125:58-61.
- [Google Scholar]
- Focal motor seizures with secondary generalization arising in the cerebellum. Case report and review of the literature. J Neurosurg. 2002;97(1):190-196.
- [Google Scholar]
- Neonatal hemifacial spasm and fourth ventricle mass. Dev Med Child Neurol. 2012;54(8):697-703.
- [Google Scholar]
- Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13(5):313-326.
- [Google Scholar]








