Translate this page into:
Systematic review and meta-analysis of studies comparing baseline D-dimer level in stroke patients with or without cancer: Strength of current evidence
*Corresponding author: Dr. Amit Agrawal, Department of Neurosurgery, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. dramitagrawal@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mishra R, Chavda VK, Moscote-Salazar LR, Atallah O, Das S, Janjua T, et al. Systematic review and meta-analysis of studies comparing baseline D-dimer level in stroke patients with or without cancer: Strength of current evidence. J Neurosci Rural Pract. 2024;15:16-28. doi: 10.25259/JNRP_379_2023
Abstract
Objectives:
D-dimer levels are increased in stroke and cancer. Cancer patients are at a higher risk of stroke. However, the evidence is unclear if high D-dimer in stroke patients can suggest the diagnosis of concomitant cancer or the development of stroke in a cancer patient. The objective is to assess the evidence available on the baseline D-dimer level in stroke patients with and without cancer.
Materials and Methods:
We conducted the systematic review and meta-analysis using the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. We searched PUBMED, Cochrane, ScienceDirect, and Scopus for potentially eligible articles published till June 2023. All the review steps were iterative and done independently by two reviewers. The Newcastle-Ottawa scale tool was used to assess the quality of included studies for case control and cohort studies and the Agency for Healthcare Research and Quality tool for cross-sectional studies. The qualitative synthesis is presented narratively, and quantitative synthesis is shown in the forest plot using the random effects model. I2 of more than 60% was considered as high heterogeneity.
Results:
The searches from all the databases yielded 495 articles. After the study selection process, six papers were found eligible for inclusion in the qualitative and quantitative synthesis. In the present systematic review, 2651 patients with ischemic infarcts are included of which 404 (13.97%) patients had active cancer while 2247 (86.02%) did not. The studies included were of high quality and low risk of bias. There were significantly higher baseline D-dimer levels in stroke patients with cancer than in non-cancer patients with a mean difference of 4.84 (3.07–6.60) P < 0.00001.
Conclusion:
D-dimer is a simple and relatively non-expensive biomarker that is increased to significant levels in stroke patients, who have cancer and therefore may be a tool to predict through screening for active or occult cancer in stroke patients.
Keywords
Cancer
D-dimer
Stroke
Prognosis
Cerebral infarction
INTRODUCTION
The D-dimer is the fibrin degradation product at minimally detectable levels in normal individuals; however, it increases in stroke patients, thrombolytic/fibrinolytic disorders, and critically sick patients.[1-3] Patients with malignancy are especially prone to develop ischemic stroke by various mechanisms including hypercoagulable state and tumor occlusion.[3-8] Various studies have reported that high plasma D-dimer levels can be seen in cancer-associated ischemic stroke patients, further supporting the assumption that hypercoagulability is a significant risk factor for developing stroke in cancer patients.[2,3,9-13] Studies have shown that abnormal D-dimer levels and multiple territories of infarcts are predictors of malignancy in a stroke patient.[2,14] In a study, the authors investigated the relationship between D-dimer levels in cancer-associated stroke patients after stroke treatment. They suggested that higher D-dimer levels were associated with poor short-term outcomes.[15] Higher levels of D-dimer have been found in the cerebral infarct in cancer patients than in non-cancer patients in several studies. In addition, studies have found that D-dimer might be an important marker in the screening of stroke in malignancy patients and in differentiating between cryptogenic stroke and stroke with determined etiology in patients with cancer.[4,16,17] A systematic review found that the D-dimer levels were elevated in the stroke and are a non-expensive marker; however, it is not specific or sensitive to the type of stroke and cannot replace the traditional clinical and radiological methods for stroke diagnosis.[18] The conflicting findings from the studies necessitate conducting a systematic review to establish the evidence on the baseline D-dimer levels in infarct in cancer versus non-cancer patients. In this systematic review and meta-analysis, we aimed to assess the evidence regarding the hypothesis that the baseline D-dimer levels are higher in patients, who present with ischemic stroke and who had a cancer diagnosis than in patients, who do not have a cancer diagnosis at the time of clinical presentation.
MATERIALS AND METHODS
The present systematic review and meta-analyses followed Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines and the meta-analyses and the Cochrane Manual of Systematic Reviews and Meta-analysis.[19] Two reviewers independently performed all the review process steps, and the third reviewer was consulted in case of any discrepancy.
Search
We searched the databases including PubMed, Scopus, Central Cochrane Registry of Controlled Trials (The Cochrane Library), and ScienceDirect until June 2023. In addition, the reference list of included studies and other relevant data in addition to potentially eligible studies were searched; the details of search terms are listed in Table 1. The search strategy was prepared and pilot-tested to maximize the specificity and sensitivity of the search terms.
| Database | Search terms |
|---|---|
| PubMed | ((“stroke”[MeSH Terms] OR “stroke”[All Fields] OR “strokes”[All Fields] OR “stroke s”[All Fields]) AND (“fibrin fragment d”[Supplementary Concept] OR “fibrin fragment d”[All Fields] OR “d dimer”[All Fields]) AND (“cancer s”[All Fields] OR “cancerated”[All Fields] OR “canceration”[All Fields] OR “cancerization”[All Fields] OR “cancerized”[All Fields] OR “cancerous”[All Fields] OR “neoplasms”[MeSH Terms] OR “neoplasms”[All Fields] OR “cancer”[All Fields] OR “cancers”[All Fields])) AND (1000/1/1:2023/6/6[pdat]) |
| Cochrane | 12 Trials matching stroke D-dimer cancer in title abstract keyword |
| Scopus | Title, abstract, keywords: Stroke D-dimer cancer |
| ScienceDirect | Title, abstract, keywords: Stroke D-dimer cancer |
Inclusion/exclusion criteria
Studies reporting on D-dimer levels in infarct patients in cancer and non-cancer were included in the study. The criteria for inclusion were original articles written in English, and the date of publication was between the date of inception and June 6, 2023. The submissions excluded were review articles, brief reports, letters, conference abstracts, and papers not written in English. Studies that did not have a non-cancer group as a comparison were excluded from the study.
Study selection and data extraction
Two reviewers did an independent screening of studies retrieved from the search sources regarding eligibility based on title and abstracts. Then, the full-length articles were retrieved. A third review author was consulted for a decision in case of any discrepancy. The data extraction was done in a pre-designed proforma. Data collected included study authors, year, country of origin, sample size, gender, age, and D-dimer values according to the groups (cancer vs. non-cancer).
Evaluation of the quality of the studies
The quality of the case–control and cohort studies was assessed using the Newcastle-Ottawa scale (NOS). The NOS mainly consists of three domains of selection, comparability, and outcome and has a subset of questions in each domain.[20] The quality of included studies was through the Newcastle-Ottawa Quality Assessment Scale, and studies with scores of 7 were considered of high methodological quality. Those with scores of 4–5 were considered moderate quality. The study quality of the cross-sectional study was done using the Agency for Healthcare Research and Quality (AHRQ), an 11-question scoring system with one score for each item.[21] A score of 0–4 indicates a high risk of bias, 5–7 means a moderate risk, and 8–11 shows a low risk of bias.[21]
Statistical analysis
The random effects analysis model was calculated using Review Manager 5.3 (Nordic Cochrane Centre, Copenhagen, Denmark). We analyzed the mean difference for each outcome using the generic method of the inverse of the variance to combine this data. We expected different units and effect estimates of reporting D-dimer levels in individual studies; and therefore, in studies where mean values were not reported, we calculated the mean values from reported effect estimates and then used mean difference as effect estimate for meta-analysis to avoid the potential problems of using standardized mean difference in such a situation.[22] The originally reported values in individual studies and converted values used for quantitative synthesis along with the references are detailed in supplementary Table S1. In the studies where units of D-dimer were not reported, we assumed the most commonly used unit of D-dimer for conversion and used in quantitative analysis and performed sensitivity analysis. Heterogeneity was assessed by calculating Chi-square (I2), with the high heterogeneity of the studies included in the analysis being above 60%.
RESULTS
Study selection
The database search retrieved 495 articles; after removing duplicates (160), 335 records were screened for eligibility, and 297 articles were excluded. The full text of the 39 articles was evaluated; 33 articles were excluded [Table 1][10,13,15,17,23-51] with reasons, and six articles [Table 2][16,52-56] were included in systematic review and meta-analyses [Figure 1]. Characteristics of the studies included are shown in Table 2. Figure 1 shows the study flow diagram of study search, screening, text retrieval, and inclusion in the qualitative and quantitative synthesis.
| Study (Year) | Reason |
|---|---|
| Alvarez-Perez et al., 2012[51] | No non-active cancer group |
| Amiri-Nikpour and Husseinzadeh, 2016[23] | Full text not available |
| Bang et al., 2023[24] | No separate group to answer research question |
| Bonnerot et al., 2016[25] | No non-active cancer group |
| Cen et al., 2023[26] | No non-active cancer group |
| Di Castelnuovo et al., 2014[27] | Patients with coronary artery disease did not answer |
| Ellis et al., 2018[28] | Study does not separately provide D-dimer data in cancer patients |
| Markus, 2020[29] | Editorial |
| Ito et al., 2018[30] | No non-active cancer group |
| Kim et al., 2021[17] | No well-defined cancer and non-cancer groups |
| Kono et al., 2012[10] | Detailed FDP values not available separate for cancer and non-cancer groups |
| Liu et al., 2021[31] | Study does not separately provide D-dimer data in cancer patients |
| Nahab et al., 2020[32] | Study does not separately provide D-dimer data in cancer patients |
| Nakajima et al., 2022[15] | No non-active cancer group |
| Nam et al., 2017[33] | No non-active cancer group |
| Nam et al., 2023[34] | No non-active cancer group |
| Nezu et al., 2018[35] | No non-active cancer group |
| Nickel et al., 2021[36] | Mix of population, no separate cancer group |
| Ohara et al., 2020[13] | Review article |
| Pan et al., 2021[38] | Study does not separately provide D-dimer data in cancer patients |
| Pan et al., 2022[37] | No non-active cancer group |
| Pieper et al., 2000[39] | Mix of population |
| Rodrigues et al., 2014[40] | Conference abstract |
| Rosenberg et al., 2020[41] | Study does not separately provide D-dimer data in cancer patients |
| Ryu et al., 2017[42] | No non-active cancer group |
| Schultz et al., 2022[43] | Conference abstract |
| Shen et al., 2020[44] | Study does not separately provide D-dimer data in cancer patients |
| Tardy et al., 1998[45] | Study does not separately provide D-dimer data in cancer patients |
| Tsushima et al., 2020[46] | No non-active cancer group |
| Wang et al., 2022[47] | No non-active cancer group |
| Wei et al., 2020[48] | No non-active cancer group |
| Yamaguchi et al., 2019[49] | No non-active cancer group |
| Zhang et al., 2019[50] | Systematic review and meta-analyses |
FDP: Fibrinogen degradation products
- Preferred reporting items for systematic reviews and meta-analysis study flow diagram, #: Number.
Quality assessment and risk of bias
The quality of the included studies, as assessed by the NOS for cohort and case–control studies, is shown in Table 3. The median quality was 7/9. Most of the included studies meet the majority criteria in the quality assessment tool. The study of Beyeler et al.[52] scored 9/11 in the AHRQ tool suggesting a low risk of bias in the study.
| S. No. | Groups | Selection | Comparability | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study Id | Representativeness of sample | Selection of the non-exposed cohort | Ascertainment of Prognostic variable | Demonstration That Outcome of Interest Was Not Present at Start of Study | Comparability of cohorts based on the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| 1. | Guo et al., 2014[16] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8/9 |
| 2. | Gon et al., 2017[53] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7/9 | |
| 3. | Sorgun et al., 2018[54] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7/9 | |
| Case Control study | Study Id | Case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls based on the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | |
| 1. | Wang et al., 2018[56] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7/9 | |
| 2. | Wang et al., 2019[55] | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7/9 | |
★Indicates that it meets criteria in Newcastle-Ottawa Scale
Characteristics of included studies
Three studies were from China[16,55,56] and one each from Switzerland, Japan, and Turkey.[52-54] All the included studies were retrospective. In the present systematic review, 2651 patients with ischemic infarcts are included of which 404 (13.97%) patients had active cancer while 2247 (86.02%) did not. In four studies,[16,52-54] infarct patients without active cancer were proportionately more than cancer patients. At the same time, the two studies[55,56] had almost equal proportions of infarct patients in the cancer and non-cancer groups. The mean age of the patients included in all the studies was more than 60 years with similar gender distribution. The characteristics of the included studies are shown in Table 4.
| Study (Author) | Country | Study type | Sample size | Age (Years) | Gender (Male/Female) | D-dimer | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | No active cancer | Cancer | No active cancer | Cancer | No active cancer | Cancer | No active cancer |
|||
| Beyeler et al., 2022[52] | Switzerland | Single-center retrospective, cross sectional | 61 | 940 | Age at admission (median, IQR) 76.8 (71.183.4) | 73.5 (62.582.3) | Female 24/61 | Female 390/940 | D-dimer in μg /L (median, IQR) 1689 (652–6852) | 701 (367.5–1524.5) |
| Gon et al., 2017[53] | Japan | Retrospective cohort | 12 | 108 | 64 (54–75) | 65 (51–74) | Female=8 | Female=43 | D-dimer (μg /mL) 6.2 (1.2–12.0) |
0.5 (0.3–0.9) |
| Guo et al., 2014[16] | China | Retrospective cohort | 98 | 430 | 70.95 ± 13.61 | 69.0 ± 12.6 | Male% 59.3 | 69.1 | D-dimer (mg/L) 7.59±10.96 | 0.66±1.83 |
| Sorgun et al., 2018[54] | Turkey | Retrospective cohort | 46 | 573 | Age, year, Mean±SD 70.70±11.04 | 69.30±13.52 | Female=17 Male=29 | Female=273 Male=300 | D-dimer, Median (Min–Max) 1,519.0 (362.0–12,487.0) |
590.5 (42.0–3,191.0) |
| Wang et al., 2019[55] | China | Retrospective case–control study | 126 | 120 | Age, years, M (IQR) 63 (41–88) | 73 (51–85 | Male% 80 (63.5) | 78 (65.0) | D-D, mg/L 5.7 (4.1–11.7) | 1.2 (0.7–6.8) |
| Wang et al., 2018[56] |
China | Retrospective case control | 61 | 76 | 64.7±11.8 | 65.4±12.6 | Male% 43 (56.6) | 32 (52.5) | D-dimer (μg/mL) 0.84±0.80 |
10.81±13.19 |
SD: Standard deviation, IQR: Interquartile range
Results of individual studies
Guo et al.[16] reported their results from 528 patients in a retrospective cohort study of which 98 had cancer while 430 did not have active cancer. The authors defined active cancer as the occurrence of cancer or recurrence within one year of the stroke diagnosis or cancer diagnosed before the stroke but with incomplete treatment. The plasma D-dimer levels in the cancer patients were much higher than in the non-cancer patients (7.59 ± 10.96 vs. 0.66 ± 1.83). The authors found significantly higher D-dimer levels in stroke patients with active cancer than in no cancer; however, there was no significant difference between the no-cancer group and inactive cancer group, i.e., the patients who had cancer before stroke but received treatment and were in complete remission. This suggests that active cancer affects the coagulation cascade, and remission from cancer results in the resolution of the coagulation abnormality. Further, the authors found that multiple infarct territories in stroke are seen more in cancer patients than in non-cancer patients. The most common malignancies resulting in stroke were gastrointestinal followed by lung and hematological malignancies. In the study, more than 60% of stroke patients with cancer had D-dimer levels above 1.55 mg/L while only 7.9% non-cancer group had D-dimer levels above this cutoff. This cutoff value had a sensitivity of 59.2% and a specificity of 91.8%. The authors further concluded that the abnormal D-dimer value was insufficient to suggest the presence of concomitant cancer. If the D-dimer values were as high as 5.5 mg/L, there should be a comprehensive search for cancer, as the specificity and positive predictive values were >93%.
Gon et al.[53] conducted a retrospective analysis of the ability of D-dimer values to predict occult cancer in 120 cryptogenic stroke patients without cancer at diagnosis. The authors defined cryptogenic stroke as a stroke without a defined identified etiology despite extensive evaluation. Of these, 12 patients (10%) had occult cancer. Among 12 patients with occult cancer, six (50%) had adenocarcinoma, five had an extensive disease in metastasis, and nine (75%) patients had ischemic lesions in multiple vascular territories. The D-dimer levels were much higher in the occult cancer group than in the non-cancer group (median 6.2 vs. median 0.5).
Sorgun et al.[54] reported the predictive value of D-dimer in a retrospective analysis of 619 stroke patients of which 46 had cancer and 573 had no active cancer. The authors defined active cancer as active cancer before stroke diagnosis. The study’s common causes of cancer leading to stroke were bladder cancer followed by gastric, lung, and hematological malignancies. The D-dimer levels were significantly higher in the cancer group than in the non-cancer group (1,519.0 vs. 590.5). The authors did not report the unit of D-dimer in their study.
Wang et al.[56] study included 137 acute ischemic stroke patients, 61 with cancer and 76 without cancer, reported higher D-dimer values in the cancer group. The most common cancers in the acute ischemic group were gastric, lung, and colorectal. In 14 patients (23.0%), cancer was detected later to the ischemic stroke with a thorough examination after admission suggesting that such stroke patients with higher D-dimer values should be screened for the presence of cancers. The cutoff value of 2.785 μg/mL of D-dimer for differentiating stroke with cancer from stroke without cancer has a sensitivity of 50.9% and specificity of 98.5%. Furthermore, patients with D-dimer higher than the cutoff values in the cancer group were more likely to have multiple territories of ischemic lesions.
Wang et al.[55] in their retrospective case–control study included 246 stroke patients (126 with cancer and 120 as control). The patients included were adults, and active cancer was defined as a new cancer diagnosis, metastasis, recurrence or ongoing cancer treatment within one year before the stroke diagnosis. The patients with confounders such as other central nervous system (CNS) diseases and cardiac, hepatic, and renal diseases were excluded. The authors found that the median levels of D-dimer were higher in the cancer group than in the control group (5.7 mg/L vs. 1.2 mg/L).
Beyeler et al.[52] in their single-center retrospective cross-sectional study included 1001 stroke patients (61 with cancer and 940 without cancer). The authors defined active cancer as a new cancer diagnosis, recurrent cancer or metastasis within six months before the stroke diagnosis and occult cancer as a cancer diagnosis within one year after the stroke diagnosis. Among the 61 patients with active malignancy at the stroke diagnosis, 22 had occult and 39 had known cancer. The median D-dimer was higher in the cancer group than in the non-cancer group (1689 μg/L vs. 701 μg/L).
Synthesis of results
The meta-analysis of the included studies using the random effects model showed that the D-dimer values were significantly higher in stroke patients with cancer than without cancer with a mean difference of 4.84 (3.07–6.60) P < 0.00001. There was a high heterogeneity of 85%. The high heterogeneity is likely due to clinical heterogeneity because of diverse populations, inclusion, exclusion criteria, confounders, and biases in the included studies. The forest plot analysis of the included studies is shown in Figure 2a. Figure 2b shows that the results of the sensitivity analysis with the study by Sorgun et al.[54] were removed, as we assumed the most common unit of D-dimer and the unit was not reported in the study; we found that the mean difference increased to 5.53 and 95% confidence interval (CI) of 3.10–7.97. Figure 2c shows that the results of the study by Beyeler et al. were removed from the analysis, as the data presented in the study were not following the normal distribution. Figure 2d shows that the results of both the studies by Sorgun et al. and Beyeler et al. were removed from the analysis, and there was further increase in the net effect estimate to mean difference of 6.58 with 95% CI of 4.00–9.16.
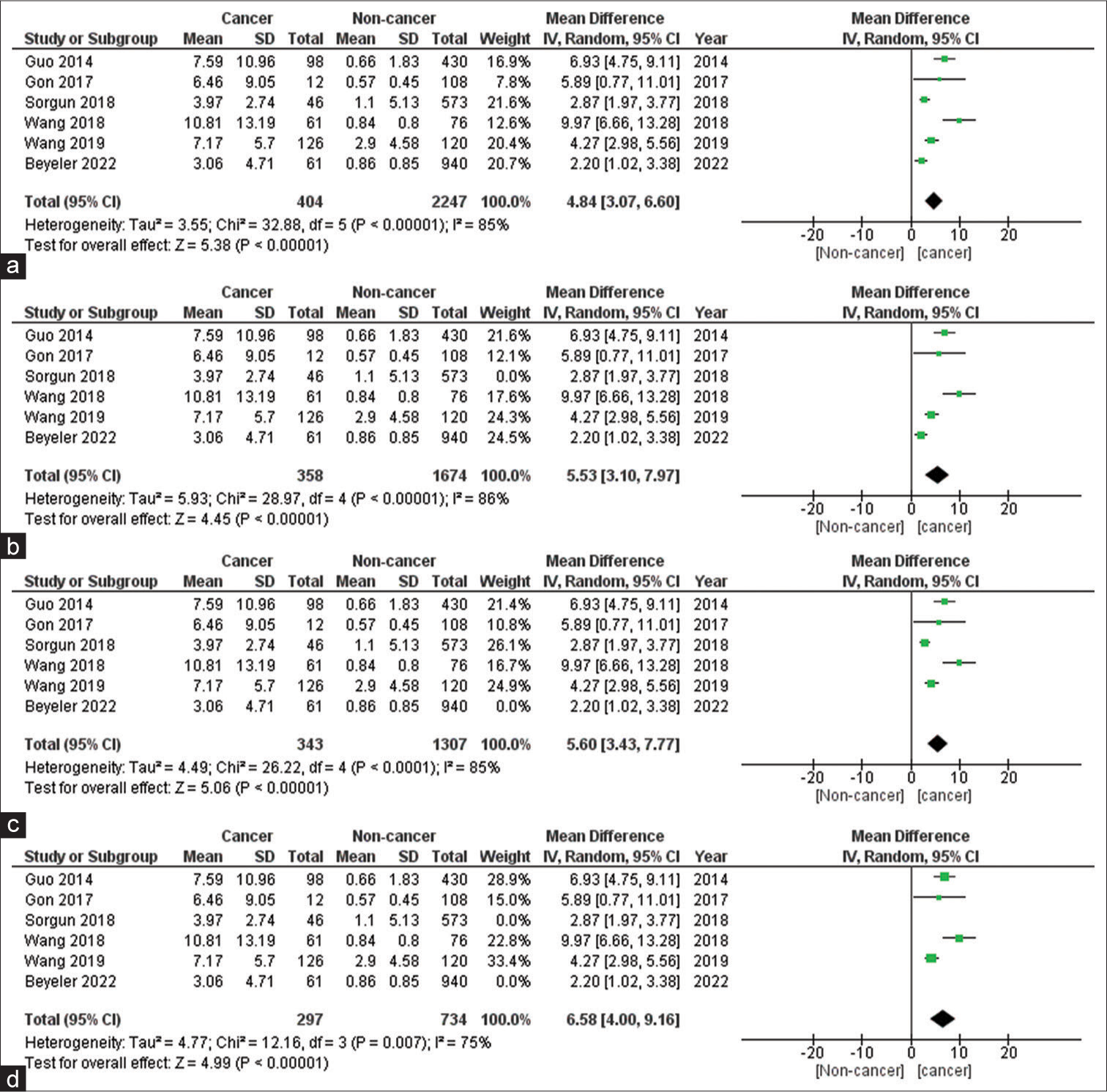
- (a) Forest plot diagram of D-dimer values in cancer and non-cancer group; (b) forest plot diagram of sensitivity analysis by removing study by Sorgun et al.; (c) forest plot diagram of sensitivity analysis by removing study by Beyeler et al.; and (d) forest plot diagram of sensitivity analysis by removing study by Sorgun et al. and Beyeler et al. SD: Standard deviation, CI: Confidence interval.
DISCUSSION
Stroke is the second leading cause of CNS involvement in cancer patients after metastatic involvement. Cancer patients are prone to stroke, and stroke in cancer happens due to altered homeostasis and activation of coagulation cascade either due to intracranial metastasis or coagulation disorder or vascular injury secondary to the chemotherapy, radiotherapy or tumor embolization.[2,57-59] Additional causes of stroke in cancer include non-bacterial thrombotic endocarditis, diffuse thrombosis of cerebral vessels or septic fungal emboli, which occur in leukemic patients, who underwent bone marrow transplantation.[2,57,58]
In the present systematic review, we have searched multiple databases and identified five retrospective cohort studies and one cross-sectional study. The pooled estimate from the included studies suggests that the D-dimer values are significantly higher in stroke patients, who had cancer than those without cancer. Although there was high heterogeneity as seen from the forest plot, the effect estimates of all the individual studies show the same direction of effect; therefore, the confidence in the results is increased. Further, some individual studies have shown that D-dimer levels above 5.5 should prompt a thorough screening of underlying cancer. The pooled effect estimate from our present review is like the cutoff values reported in other studies.
The differences in the studies could be due to the methodology with most studies being retrospective and the variation in the cancer studies. It has been found that bleeding was most likely in leukemia patients while infarction was the most common cerebrovascular finding in carcinoma patients.[60] In an autopsy study, only 7.4% had clinical symptoms while 14.6% had pathological evidence of cerebrovascular disease.[60]
In the study by Ryu et al., the authors reported on the predictive value of D-dimer in predicting cerebral infarction in critically ill cancer patients (43 infarcts vs. 43 noninfarcts).[42] The study was retrospective and included only cancer patients. Patients were evaluated with diffusion-weighted magnetic resonance imaging based on clinical suspicion and found that there was no significant difference in the D-dimer among the patients with infarct or non-infarct, but D-dimer levels >8.89 μg/mL were more associated with the cryptogenic mechanisms of the stroke than the determined mechanisms of the stroke.[42]
Strengths
A detailed and iterative process reduces the bias. We performed the sensitivity analysis by removing studies where conversion of data from the reported data could result in erroneous conclusions, and we found in the sensitivity analysis a net addition to the effect estimate of the mean difference. This adds strength and generalizability to the results of the analysis. Further, we have estimated the mean difference to avoid the potential problems of using standardized mean difference by converting the data reported in the individual studies to common units, mean, and standard difference. This also adds to the generalizability and strength of the results obtained.
Future implications and limitations
The stroke itself is associated with increased levels of D-dimer.[51,61] In the literature, it is published that the mortality from stroke in cancer patients is almost double that of stroke in cancer patients.[62-64] The present review is critical from a clinical viewpoint because it is helpful from the patient care perspective that cancer patients are prone to stroke, and many patients currently with stroke are harboring occult or active cancer and should be actively screened. Both these dimensions are essential to explore for better patient care and outcomes. Two different study designs are likely to provide answers to these questions. In our current review, all the included studies included patients with stroke and then looked at the presence or absence of cancer. The optimal methodology of meta-analysis of biomarker-based studies is still evolving. D-dimer is a very common thrombotic biomarker, which can be raised both in stroke and cancer. Therefore, the clinical significance of raised D-dimer in stroke patients with cancer may be a potential biomarker to differentiate cancer from non-cancer patients with stroke. Due to the heterogeneity of studies optimal threshold level of D-dimer (cut-off value) could not be ascertained in this study. As the included studies do not have a group of cancer patients without stroke for comparison, it cannot be stated conclusively whether the elevated D-dimer levels are because of the stroke or cancer. Further, the included studies have not performed the cancer screening comprehensively on all the patients, and this selection bias might influence the results obtained. The other scenario is where cancer patients are included, and then the comparison is drawn between the stroke and non-stroke groups. As both the disease processes are different but interact, several other parameters might affect the outcome of the interest being measured. Consequently, a more extensive study with multiple groups will be more helpful in addressing these questions.
CONCLUSION
Baseline D-dimer levels are significantly higher in stroke patients with cancer than in non-cancer patients with stroke. There is a hypercoagulable state in cancer patients, and increased D-dimer levels contribute to an increased risk of stroke in cancer patients. D-dimer is a readily available, less expensive screening biomarker in stroke patients that may suggest screening for cancer. The strength of evidence is low due to fewer studies, and much larger prospective studies will provide a more substantial evidence based on baseline D-dimer in stroke patients with and without cancer.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- D-dimer testing in laboratory practice. Clin Chem. 2011;57:1256-62.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemic stroke in cancer patients with and without conventional mechanisms: A multicenter study in Korea. Stroke. 2010;41:798-801.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke and cancer: The importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029-34.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemic stroke and cancer: Stroke severely impacts cancer patients, while cancer increases the number of strokes. J Clin Neurol. 2011;7:53-9.
- [CrossRef] [PubMed] [Google Scholar]
- The long-term effect of cancer on incident stroke: A nationwide population-based cohort study in Korea. Front Neurol. 2019;10:52.
- [CrossRef] [PubMed] [Google Scholar]
- New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology. 2018;90:e2025-33.
- [CrossRef] [PubMed] [Google Scholar]
- Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291-300.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of haemorrhagic and ischaemic stroke in patients with cancer: A nationwide follow-up study from Sweden. Eur J Cancer. 2012;48:1875-83.
- [CrossRef] [Google Scholar]
- Characteristics of cryptogenic stroke in cancer patients. Ann Clin Transl Neurol. 2016;3:280-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int. 2012;12:468-74.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann Neurol. 2018;83:873-83.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanisms of ischemic stroke in patients with cancer: A prospective study. Ann Neurol. 2021;90:159-69.
- [CrossRef] [PubMed] [Google Scholar]
- The emerging value of serum D-dimer measurement in the work-up and management of ischemic stroke. Int J Stroke. 2020;15:122-31.
- [CrossRef] [PubMed] [Google Scholar]
- Clues to occult cancer in patients with ischemic stroke. PLoS One. 2012;7:e44959.
- [CrossRef] [PubMed] [Google Scholar]
- Post-treatment plasma D-dimer levels are associated with short-term outcomes in patients with cancer-associated stroke. Front Neurol. 2022;13:868137.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of plasma (D)-dimer levels for cancer-related stroke: A 3-year retrospective study. J Stroke Cerebrovasc Dis. 2014;23:e249-54.
- [CrossRef] [PubMed] [Google Scholar]
- The role of factor Xa-independent pathway and anticoagulant therapies in cancer-related stroke. J Clin Med. 2021;11:123.
- [CrossRef] [PubMed] [Google Scholar]
- Is D-dimer helpful in evaluating stroke patients? A systematic review. Acta Neurol Scand. 2009;119:141-50.
- [CrossRef] [PubMed] [Google Scholar]
- The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;88:105906.
- [CrossRef] [PubMed] [Google Scholar]
- The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Last accessed on 2023 Jul 10]
- [Google Scholar]
- Celiac disease. 2004. Agency for Healthcare Research and Quality (US). Available from: https://sep.evidencereports/technologyassessments,no.104.appendixD quality assessment forms.2014 [Last accessed on 2023 Jun 13]
- [Google Scholar]
- Standardized or simple effect size: What should be reported? Br J Psychol. 2009;100:603-17.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the changes of D-Dimer and FDP serum levels in ischemic brain stroke patients with and without malignancy. J Glob Pharma Technol. 2016;8:240-5.
- [Google Scholar]
- Circulating extracellular-vesicle-incorporated micrornas as potential biomarkers for ischemic stroke in patients with cancer. J Stroke. 2023;25:251-65.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral ischemic events in patients with pancreatic cancer: A retrospective cohort study of 17 patients and a literature review. Medicine (Baltimore). 2016;95:e4009.
- [CrossRef] [PubMed] [Google Scholar]
- The investigation on the hypercoagulability of hepatocellular carcinoma-related cerebral infarction with thromboelastography. Brain Behav. 2023;13:e2961.
- [CrossRef] [PubMed] [Google Scholar]
- Elevated levels of D-dimers increase the risk of ischaemic and haemorrhagic stroke, Findings from the EPICOR Study. Thromb Haemost. 2014;112:941-6.
- [CrossRef] [PubMed] [Google Scholar]
- Coagulation markers and echocardiography predict atrial fibrillation, malignancy or recurrent stroke after cryptogenic stroke. Medicine (Baltimore). 2018;97:e13830.
- [CrossRef] [PubMed] [Google Scholar]
- D-dimer in ischemic stroke, and insights from HEADPOST into swallowing problems and outcome after stroke. Int J Stroke. 2020;15:121.
- [CrossRef] [PubMed] [Google Scholar]
- Changes in serial D-dimer levels predict the prognoses of trousseau's syndrome patients. Front Neurol. 2018;9:528.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of the markers of coagulation and hemostatic activation profile in the management of embolic strokes of undetermined source. J Stroke Cerebrovasc Dis. 2021;30:105592.
- [CrossRef] [PubMed] [Google Scholar]
- Markers of coagulation and hemostatic activation aid in identifying causes of cryptogenic stroke. Neurology. 2020;94:e1892-9.
- [CrossRef] [PubMed] [Google Scholar]
- D-dimer as a predictor of early neurologic deterioration in cryptogenic stroke with active cancer. Eur J Neurol. 2017;24:205-11.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical significance of D-dimer levels during acute period in ischemic stroke. Thromb J. 2023;21:55.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of D-dimer levels for short-term or long-term outcomes in cryptogenic stroke patients. J Neurol. 2018;265:628-36.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnoses and outcomes of emergency patients with an elevated D-dimer over the next 90 days. Am J Med. 2021;134:260-6.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Significance of D-dimer in acute ischemic stroke patients with large vessel occlusion accompanied by active cancer. Front Neurol. 2022;13:843871.
- [CrossRef] [PubMed] [Google Scholar]
- Development and validation of a nomogram for lower extremity deep venous thrombosis in patients after acute stroke. J Stroke Cerebrovasc Dis. 2021;30:105683.
- [CrossRef] [PubMed] [Google Scholar]
- Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. J Gerontol A Biol Sci Med Sci. 2000;55:M649-57.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke as first signal of cancer-stroke unit retrospective study. Ann Oncol. 2014;25:iv492.
- [CrossRef] [Google Scholar]
- D-dimer and body CT to identify occult malignancy in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:105366.
- [CrossRef] [PubMed] [Google Scholar]
- D-dimer levels and cerebral infarction in critically ill cancer patients. BMC Cancer. 2017;17:591.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of D-dimer as an alternative biomarker of thrombosis in heartmate 3 recipients. J Heart Lung Transpl. 2022;41:S463-4.
- [CrossRef] [Google Scholar]
- D-dimer and diffusion-weighted imaging pattern as two diagnostic indicators for cancer-related stroke: A case-control study based on the STROBE guidelines. Medicine (Baltimore). 2020;99:e18779.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of D-dimer ELISA test in elderly patients with suspected pulmonary embolism. Thromb Haemost. 1998;79:38-41.
- [CrossRef] [PubMed] [Google Scholar]
- D-dimer and C-reactive protein as potential biomarkers for diagnosis of trousseau's syndrome in patients with cerebral embolism. J Stroke Cerebrovasc Dis. 2020;29:104534.
- [CrossRef] [PubMed] [Google Scholar]
- In patients with acute ischemic stroke and cancer: The shorter interval, the higher D-dimer. Asian Pac J Cancer Prev. 2022;23:2375-8.
- [CrossRef] [PubMed] [Google Scholar]
- Profiling of the risk factors and designing of a model to identify ischemic stroke in patients with non-hodgkin lymphoma: A multicenter retrospective study. Eur Neurol. 2020;83:41-8.
- [CrossRef] [PubMed] [Google Scholar]
- Active cancer and elevated D-dimer are risk factors for in-hospital ischemic stroke. Cerebrovasc Dis Extra. 2019;9:129-38.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic role of early D-dimer level in patients with acute ischemic stroke. PLoS One. 2019;14:e0211458.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency and mechanism of ischemic stroke associated with malignancy: A retrospective series. Eur Neurol. 2012;68:209-13.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a score for prediction of occult malignancy in stroke patients (Occult-5 Score) J Stroke Cerebrovasc Dis. 2022;31:106609.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma D-dimer levels and ischaemic lesions in multiple vascular regions can predict occult cancer in patients with cryptogenic stroke. Eur J Neurol. 2017;24:503-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors, biomarkers, etiology, outcome and prognosis of ischemic stroke in cancer patients. Asian Pac J Cancer Prev. 2018;19:649-53.
- [Google Scholar]
- Clinical and imaging characteristics of malignant tumor concurrent with stroke. Cancer Biother Radiopharm. 2019;34:504-10.
- [CrossRef] [PubMed] [Google Scholar]
- D-dimer >2.785 mug/ml and multiple infarcts >/=3 vascular territories are two characteristics of identifying cancer-associated ischemic stroke patients. Neurol Res. 2018;40:948-54.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrovascular complications in patients with cancer. Semin Neurol. 2010;30:311-9.
- [CrossRef] [PubMed] [Google Scholar]
- Major cerebral infarction from tumor embolus. Stroke. 1986;17:555-7.
- [CrossRef] [PubMed] [Google Scholar]
- The hypercoagulable state of malignancy: Pathogenesis and current debate. Neoplasia. 2002;4:465-73.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrovascular complications in patients with cancer. Medicine (Baltimore). 1985;64:16-35.
- [CrossRef] [PubMed] [Google Scholar]
- Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. Arch Intern Med. 2002;162:2589-93.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke risk factor, pattern and outcome in patients with cancer. Acta Neurol Scand. 2006;114:378-83.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factor, pattern, etiology and outcome in ischemic stroke patients with cancer: A nested case-control study. Cerebrovasc Dis. 2007;23:181-7.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke in patients with cancer: Incidence and etiology. Neurology. 2004;62:2025-30.
- [CrossRef] [PubMed] [Google Scholar]
| Study (Author) | Sample size | Unit | D-Dimer | Remarks for conversion calculations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | No active cancer | Cancer | No active cancer | ||||||||||||
| Beyeler, 2022[52] | 61 | 940 | Reported: D-dimer in μg/L (median, IQR) | 1689 (652-6852) | 1689 | 652 | 6852 | 701 (367.5-1524.5) | 701 | 367.5 | 1524.5 | The values were converted from µg/L unit to mg/L and then the median values were converted to mean and standard deviation using the formula below. The text in the manuscript contains the values reported by authors, while the values in the forest plot are computed mean values for quantitative analysis. | |||
| Reference: | |||||||||||||||
| S.P. Hozo, B. Djulbegovic, and I. Hozo, BMC Medical Research Methodology 2005,5:13 | |||||||||||||||
| Converted: D-dimer in mg/L (median, IQR) | 1.689 | 0.652 | 6.85 | 0.7 | 0.368 | 1.5245 | |||||||||
| Converted: D-dimer (mg/L) (mean±standard deviation) | 3.06±4.71 | 0.864±0.859 | |||||||||||||
| Gon, 2017[53] | 12 | 108 | Reported: D-dimer (μg/mL) (median, IQR) | 6.2 (1.2–12.0) | 6.2 | 1.2 | 12 | 0.5 (0.3–0.9) | 0.5 | 0.3 | 0.9 | The values were converted from µg/mL unit to mg/L and then the median values were converted to mean and standard deviation using the formula below. The text in the manuscript contains the values reported by authors, while the values in the forest plot are computed mean values for quantitative analysis. | |||
| Reference: | |||||||||||||||
| S.P. Hozo, B. Djulbegovic, and I. Hozo, BMC Medical Research Methodology 2005,5:13 | |||||||||||||||
| Converted: D-dimer (mg/L) (median, IQR) | 6.2 | 1.2 | 12 | 0.5 | 0.3 | 0.9 | |||||||||
| Converted: D-dimer (mg/L) (mean±standard deviation) | 6.46±9.05 | 0.57±0.45 | |||||||||||||
| Guo, 2014[16] | 59 | 430 | Reported: D-dimer (mg/L) (mean±standard deviation) | 5.70±9.63 | 5.7 | 9.63 | 0.66±1.83 | 0.66 | 1.83 | The two means of the SRS group and the Hospital database group was combine using cochrane’s formula. | |||||
| Reference: | |||||||||||||||
| Altman DG, Machin D, Bryant TN and Gardner MJ. (2000) Statistics with Confidence Second Edition. BMJ Books ISBN 0 7279 1375 1. p. 28-31 | |||||||||||||||
| Higgins JPT, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. | |||||||||||||||
| Sorgun, 2018[54] | 46 | 573 | Reported: D-dimer, Median (Min-Max) | 1,519.0 (362.0-12,487.0) | 1519 | 362.1 | 12487 | 590.5 (42.0-3,191.0) | 590.5 | 42 | 191 | Most common unit of D-dimer is assumed and median is converted to mean with standard deviaion | |||
| Converted: D-dimer (mg/L) (mean±standard deviation) | 2.74±3.03 | ||||||||||||||
| Wang, 2019[55] | 126 | 120 | Reported: D-dimer, mg/L (median, IQR) | 5.7 (4.1–11.7) | 5.7 | 4.1 | 11.7 | 1.2 (0.7–6.8) | 1.2 | 0.7 | 6.8 | The text in the manuscript contains the values reported by authors, while the values in the forest plot are computed mean values for quantitative analysis. | |||
| Reference: | |||||||||||||||
| S.P. Hozo, B. Djulbegovic, and I. Hozo, BMC Medical Research Methodology 2005,5:13 | |||||||||||||||
| Converted: D-dimer (mg/L) (mean±standard deviation) | 7.17±5.7 | 2.9±4.58 | |||||||||||||
| Wang, 2018[56] | 61 | 76 | Reported: D-dimer (μg/ml)(mean±standard deviation) | 10.81±13.19 | 10.81 | 13.19 | 0.84±0.80 | 0.84 | 0.8 | The mean and SD for d-dimer calculated in mg/L units from µg/mL for the purpose of forest plot analysis. | |||||
| Converted: D-dimer (mg/L)(mean±standard deviation) | 10.81±13.19 | 10.81 | 13.19 | 0.84±0.80 | 0.84 | 0.8 | |||||||||
IQR: Interquartile range, SD: Standard deviation







