Translate this page into:
White Matter Changes in Corpus Callosum in a Patient with Idiopathic Normal Pressure Hydrocephalus
Address for correspondence: Dr. Premkumar Nattanmai, Department of Neurology, University of Missouri, 1 Hospital Drive, Columbia, MO 65211, USA. E-mail: nattanmaip@health.missouri.edu
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Idiopathic normal pressure hydrocephalus (INPH) is characterized by the clinical triad of gait and cognitive dysfunction and urinary incontinence. Ventriculoperitoneal (VP) shunting is often required for treatment. Review of literature shows few case reports discussing benign magnetic resonance imaging (MRI) T2 hyperintense changes in the corpus callosum of NPH patients after shunting due to mechanical compression of the middle and posterior regions of the body against falx cerebri leading to ischemic demyelination. These changes can be a delayed phenomenon and may interfere with clinical evaluation and may lead to unnecessary procedures and investigations. We present a patient with NPH who was admitted to the neurocritical care unit in coma with quetiapine and trazodone overdose. Diffuse changes in the body of the corpus callosum were seen on MRI suspicious for acute vasogenic edema due to drug overdose. However, it was later determined to be due to the VP shunting for the NPH. We report this case to raise the awareness of neuroimaging changes in patients with NPH who have VP shunting.
Keywords
Corpus callosum
normal pressure hydrocephalus
ventriculoperitoneal shunting
white matter changes
INTRODUCTION
Idiopathic normal pressure hydrocephalus (INPH) is diagnosed when patients present with symptomatic triad of cognitive dysfunction, gait disturbances, and urinary incontinence. Magnetic resonance imaging (MRI) is the imaging modality of choice which may show thinning and bowing of the corpus callosum and an empty sella. MRI also helps differentiate between communicating hydrocephalus from obstructive hydrocephalus. Ventriculoperitoneal (VP) shunting, if performed early, improves patient's gait, urinary incontinence, and cognitive dysfunction.[1] Rarely, patients develop extensive isolated corpus callosum magnetic resonance T2 fluid-attenuated inversion recovery (FLAIR) signal changes after VP shunting which is considered secondary to ischemic demyelination from chronic mechanical compression of this commissure by falx cerebri.[2345] Review of literature shows no significant symptoms of disconnection syndrome despite these extensive white matter changes affecting the corpus callosum.
CASE REPORT
A 64-year-old Caucasian female was found down at home with an empty bottle of quetiapine. Her last known normal was 2 h before admission. Medical history included hypertension, depression, hypothyroidism, insomnia, and INPH status postright VP shunting 2 years before presentation with a shunt revision 1 year later. Her medications included quetiapine, sertraline, trazodone, labetalol, hydrochlorothiazide, and levothyroxine. On arrival to our hospital, she was hypotensive with systolic blood pressure in the mid-80s. Her Glasgow coma scale score was 8. She had shallow breathing and required intubation for airway protection. Her neurological examination showed 3 mm sluggishly reactive pupils bilaterally, intact vestibulo-ocular, corneal, cough reflexes, withdrawing all four extremities to pain. She had generalized hyperreflexia with upgoing plantars bilaterally. Her computed tomography head showed communicating hydrocephalus with the VP shunt to be in good position. X-ray shunt series was unremarkable. She received naloxone in the emergency room with no improvement. She had a serum blood sugar of 172 mg/dL (normal 74–106 mg/dL). There was no history of tobacco or alcohol use or prior suicide attempt. Electrocardiogram showed QTc interval of 437 ms. She was admitted to the neurointensive care unit for severe encephalopathy. Her workup included a negative urine drug screen, a normal complete blood count, a comprehensive metabolic panel, cardiac enzymes, an ammonia of 16 mcmol/L (normal 11–51 mcmol/L), a B12 of 418 pg/mL (normal 211–946 pg/mL), and a thyroid-stimulating hormone of 0.862 mU/mL (normal 0.270–4.20 mU/mL). Cerebrospinal fluid analysis showed elevated protein (134 mg/dL; normal 15–45 mg/dL), glucose (80 mg/dL; normal 40–70 mg/dL), and white blood cell (1/mcL; normal 0–5/mcL). Continuous electroencephalography showed a mild diffuse encephalopathy with 6–7 Hz posterior rhythm and normal sleep architecture and reactivity. MRI of the brain showed extensive patchy, T2 hyperintensity, and swelling of the body and to a lesser extent splenium and genu of the corpus callosum [Figure 1] which were not visualized in the prior MRI of the brain axial T2 FLAIR before the surgery [Figure 2a] and in the CT scan of the head done immediately after the VP shunt procedure [Figure 2b]. We obtained serum levels of Quetiapine and trazodone and found them elevated (Quetiapine 2218 ng/dL [normal range 100-1000 ng/dL]; Trazodone 835.4 ng/dL [normal range <250 ng/dL]). She improved over several days and was discharged 6 days after admission with outpatient physical therapy. A repeat CT head [Figure 3] 1 year from admission continued to show hypodensity of the corpus callosum. Clinically, there has been no new symptoms of disconnection or progression of cognitive and gait disturbances.
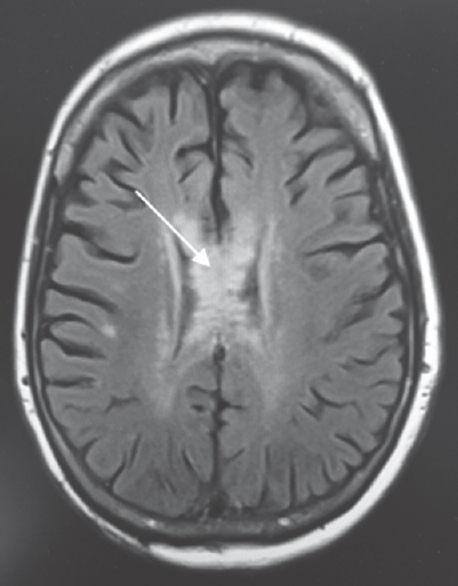
- Fluid-attenuated inversion recovery sequence of magnetic resonance imaging. Fluid-attenuated inversion recovery changes are seen in the body, splenium, and genu of the corpus callosum (arrow)
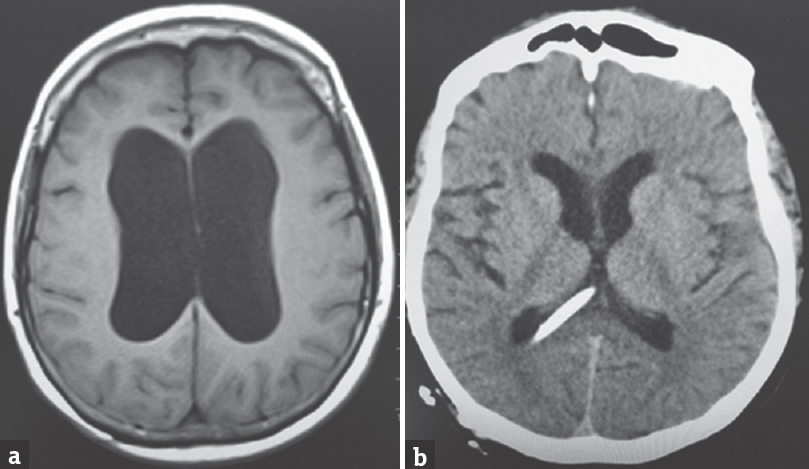
- Initial magnetic resonance imaging of the brain axial T2 fluid-attenuated inversion recovery image showing the communicating hydrocephalus with thinning and effacement of the corpus callosum (a) computed tomography of the head axial cut postprocedure showing ventriculoperitoneal shunt tip and resolution of hydrocephalus (b)
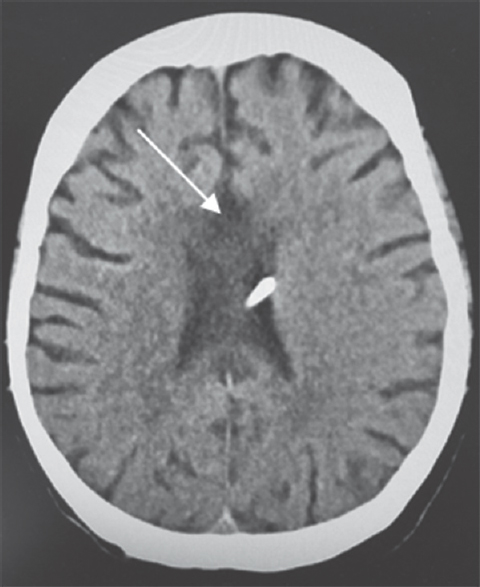
- Computed tomography of the head. Follow-up computed tomography at 1 year shows hypodensity changes in the body, genu, and splenium of the corpus callosum (arrow)
DISCUSSION
The corpus callosum is the largest commissure connecting the cerebral hemispheres. It is affected by a wide variety of central nervous system pathologies. Reversible MRI T2 hyperintensity white matter changes in the corpus callosum have been reported with drug toxicities, demyelinating disorders, systemic infections, and status epilepticus.[67] Hydrocephalus has also been reported to cause T2 hyperintense changes.[23458] We present here a case of the corpus callosum hyperintensity changes in setting of communicating hydrocephalus and medication overdose.
Few cases with isolated MRI T2 FLAIR hyperintensity changes in the corpus callosum in NPH were reported after VP shunting and attributed to long-standing mechanical compression by the falx cerebri resulting in ischemic demyelination.[239] Xiong et al. showed a decreased number of capillaries in the body of the corpus callosum in a rat model simulating making it susceptible to changes in fluid dynamics and metabolism.[69] In our patient, the initial imaging failed to show the changes in the corpus callosum possibly due to effacement and thinning of the majority of this commissure by dilated lateral ventricles. The changes became evident once the hydrocephalus was decompressed. She was admitted to the neurointensive care unit in coma with possible seroquel and trazodone overdose which made us suspect acute demyelination in the corpus callosum. Follow-up CT head at 1 year showed persistent changes in the corpus callosum. Thus, we concluded that these changes were secondary NPH and not due to overdose as the changes are mostly transient with the latter.
Given the unawareness of these post-VP shunt changes and the growing knowledge of increasing toxic-metabolic, infectious, and inflammatory disorders affecting the corpus callosum, patients can be potentially exposed to harmful procedures such as brain biopsies, lumbar punctures, and workup for toxic or metabolic leukoencephalopathies when these benign chronic changes are mistaken for acute vasogenic edema or demyelination.[3] Lane et al., in their case series, showed that 8.3% of obstructive hydrocephalus patients had diffused anterior to posterior corpus callosum white matter changes unrelated to iatrogenic shunt catheter injury.[4] Spreer et al. showed 7 out of 79 CT scans in postshunting patients with hypodense lesions of variable origin in the anterior corpus callosum.[8]
CONCLUSIONS
Isolated neuroradiological changes in the corpus callosum in shunted hydrocephalus patients may not be of clinical significance despite the extensive MRI T2 hyperintensity white matter changes. If present, may create a clinical dilemma in patients presenting with toxic metabolic encephalopathy and predispose them to undergo unnecessary procedures and treatments.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Clinical outcomes after ventriculoatrial shunting for idiopathic normal pressure hydrocephalus. Clin Neurol Neurosurg. 2016;143:34-8.
- [Google Scholar]
- Corpus callosal signal changes in patient with obstructive hydrocephalus after ventriculoperitoneal shunting. Neurologia. 2005;20:692-3.
- [Google Scholar]
- Corpus callosum changes following shunting for hydrocephalus: Case report and review of the literature. Clin Neurol Neurosurg. 2005;107:351-4.
- [Google Scholar]
- Corpus callosal signal changes in patients with obstructive hydrocephalus after ventriculoperitoneal shunting. AJNR Am J Neuroradiol. 2001;22:158-62.
- [Google Scholar]
- Corpus callosal changes associated with hydrocephalus: A report of two cases. Neurosurgery. 1997;41:488-93.
- [Google Scholar]
- Reversible splenial lesion with restricted diffusion in a wide spectrum of diseases and conditions. J Neuroradiol. 2006;33:229-36.
- [Google Scholar]
- Reversible demyelinisation of corpus callosum in the course of Marchiafava-Bignami disease. Neurol Neurochir Pol. 2006;40:156-61.
- [Google Scholar]
- Lesions of the corpus callosum in hydrocephalic patients with ventricular drainage – A CT-study. Acta Neurochir (Wien). 1996;138:174-8.
- [Google Scholar]
- An animal model of corpus callosum impingement as seen in patients with normal pressure hydrocephalus. Invest Radiol. 1993;28:46-50.
- [Google Scholar]






