Translate this page into:
Was it a case of acute disseminated encephalomyelitis? A rare association following dengue fever
Address for correspondence: Dr. Ritu Karoli, Department of Medicine, Era's Lucknow Medical College, Sarfarazganj, Hardoi Road, Lucknow, Uttar Pradesh - 226 003, India. E-mail: ritu.karoli@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Dengue infection, caused by a flavivirus is endemic in more than hundred countries, mostly in the developing world. Recent observations indicate that the clinical profile of dengue is changing, and that atypical manifestations are being reported more frequently. The exact incidence of various neurological complications is variable. Acute disseminated encephalomyelitis (ADEM) is a neurological manifestation rarely described in association with dengue. We present a patient, 32-year-old female who was diagnosed as a case of dengue fever initially after an acute febrile illness and two weeks later admitted in emergency with seizures and altered sensorium. Although MRI did not show typical lesions suggestive of ADEM, the lag period between initial dengue infection and neurological manifestations and complete recovery with methyl prednisolone point towards immune mediated demyelinating illness.
Keywords
Acute disseminated encephalomyelitis
atypical manifestations
dengue
neurological complication
Introduction
Dengue infection, caused by a flavivirus is endemic in more than hundred countries, mostly in the developing world. Recent observations indicate that the clinical profile of dengue is changing, and that atypical manifestations are being reported more frequently. Nervous system involvement in dengue infection is seen with the serotypes 2 and 3. The exact incidence of various neurological complications is variable. In countries endemic to dengue, it will be prudent to investigate for dengue infection in patients with fever and acute neurological manifestations. The exact incidence of various neurological complications of dengue infection is unknown. The reported incidence of encephalopathy and encephalitis, the most common neurological complications of dengue, has been found to vary between 0.5%[1] and 6.2%.[2]
In dengue infection, pathogenesis of neurological complications and the contribution of viral and host factors are not well understood and can be related to direct viral invasion; systemic complications related to dengue infection; or immune-mediated, autoimmune reaction secondary to dengue infection.[3] Acute disseminated encephalomyelitis (ADEM) is an acute demyelinating disorder of central nervous system and characterized by multifocal white matter involvement. ADEM as a neurological manifestation has been rarely described in association with dengue.[456] Possibly a T cell mediated autoimmune response to myelin basic protein underlies its pathogenesis and most patients improve with methylprednisolone with favorable long term prognosis.
We present a patient, 32-year-old female who was diagnosed as a case of dengue fever initially after an acute febrile illness and two weeks later admitted in emergency with seizures and altered sensorium.
Case Report
A 32-year-old female from rural background presented in medical outpatient department with complaints of high grade fever with chills, myalgias and arthralgia along with abdominal pain and occasional vomiting for 5-6 days. She was febrile and had tachycardia. Rest of the examination was normal. Her investigations showed thrombocytopenia (platelet count 60,000/cmm) and positive serology for acute dengue infection. The serum IgM antibody to dengue virus was positive. The serum IgM antibody were analyzed by ELISA method using IgM ELISA Kit by Panbio Invernis Medical Innovations, Australia. We could not assess type of dengue infection due to technical constraints. She was treated conservatively and shown improvement. About two weeks later she returned in emergency ward in state of altered consciousness with history of two episodes of generalized tonic clonic convulsions. She had normal vitals and general examination except mild pallor. Neurological examination revealed that she was drowsy but arousable and disoriented. There was no apparent cranial nerve palsy, motor deficit or signs of meningeal irritation. Ophthalmological evaluation did not show any abnormality. Her hematological and biochemical parameters were also within normal limits.
Cerebrospinal fluid examination (CSF) revealed total leukocyte count 320/cmm with 85% lymphocytosis, CSF proteins 200 mg/dl. CSF glucose, chloride and adenosine deaminase were within normal limits. Gram stain and microscopy for Mycobacterium tuberculosis was negative. Magnetic resonance imaging (MRI) brain revealed patchy areas of hyper intense signals on FlAIR (fluid attenuated inversion recovery sequence) images [Figure 1a–d]. ELISA for HIV was negative. Serology for viral hepatic markers, Japanese encephalitis, Epstein Barre virus and HSV-1 was also negative. She was treated with intravenous methyl prednisolone 1 gm once daily for five days then oral prednisolone 40 mg/day tapered over 3-4 weeks. Rapid clinical improvement was noticed. The repeat MRI after two weeks also showed almost complete resolution of patchy demyelinating lesions [Figure 2].
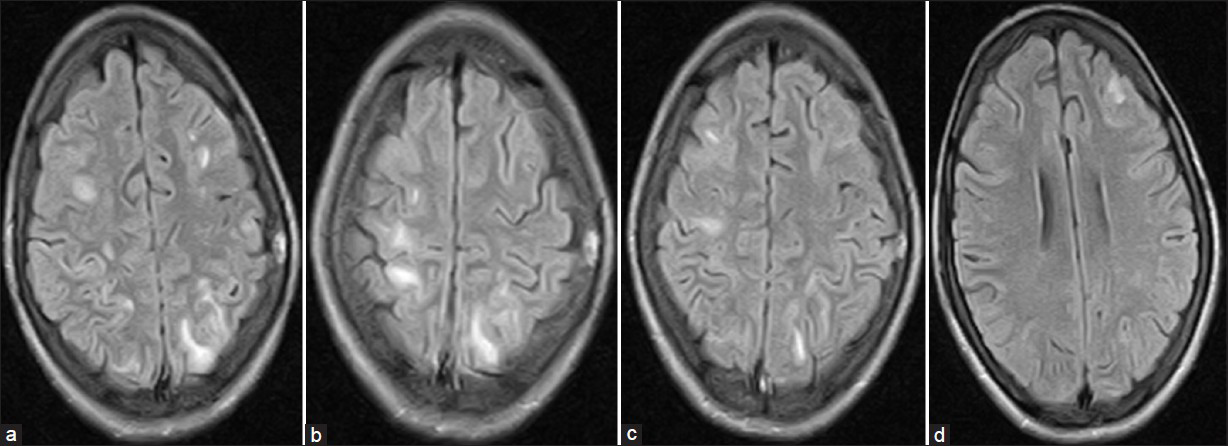
- MRI brain (Flair) showing hyper intense patchy areas of demyelination at the time of second presentation
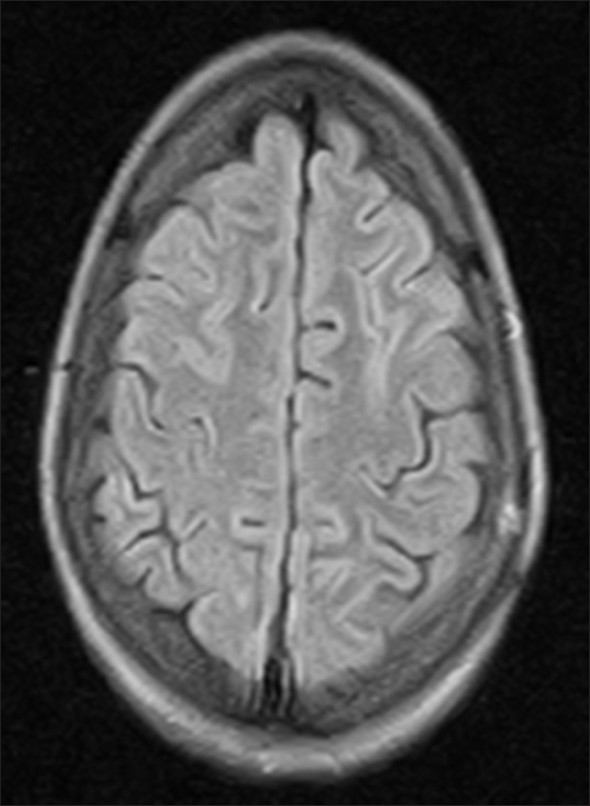
- Repeat MRI brain after two weeks showing complete resolution
Discussion
The neurological complications of dengue infection can be classified into three categories. Neurotropic effect of virus results in encephalitis, meningitis, myositis and myelitis: Systemic complications manifest as encephalopathy, stroke or hypokalemic paralysis while post infectious immune-mediated complications of dengue include acute disseminated encephalomyelitis, neuromyelitis optica, optic neuritis and Guillain-Barre syndrome.[3]
The dengue virus may lead to immune-mediated injury and acute demyelinating illness. The molecular mimicry may be responsible for immunogenic injury as in brachial neuritis and in Guillaine-Barre syndrome. The good response to steroids in our patient also suggests role of inflammation and immune-mediated injury in the pathogenesis of acute demyelinating illness. Acute disseminated encephalomyelitis (ADEM) also known as post infectious encephalomyelitis is an acute demyelinating disease of the central nervous system (CNS) that typically presents as a monophasic disorder associated with multifocal neurological symptoms and disability. It may follow vaccination in children or infection. Viral infection like measles, rubella, influenza, Epstein bar, HIV, herpes, cytomegalovirus and West Nile virus have been implicated in the causation. Among bacteria, group A hemolytic streptococcus, Mycoplasma pneumoniae, Chlamydia, Rickettesia and Leptospira have been shown to cause ADEM.
Most cases of ADEM begin about 7-14 days after infection or vaccination. The hallmark of clinical features of ADEM is the development of focal or multifocal neurological disorder. Initial clinical feature include encephalopathy ranging from lethargy to coma and focal or multiple neurological signs due to involvement of basal ganglia, thalamus, cranial nerves or spinal cord. Seizures may occur. The mechanism of ADEM is not completely understood. It may results from a transient autoimmune response towards myelin or other self antigens possibly via molecular mimicry or by non-specific activation of auto-reactive clones.[7] It has been proposed that peptides from microbial proteins that have sufficient structural similarity with the hosts self peptides can activate T-lymphocytes. T-lymphocytes when activated infiltrate the central nervous system and result in additional mono-nuclear cells to cross the blood brain barrier, leading to inflammation and demyelination.[8]
ADEM following dengue infection is extremely rare; there are only few reports of ADEM due to dengue infection that have been published. Sundaram et al.,[5] have documented an autopsy-confirmed case of ADEM who had hemorrhagic foci in the demyelinating lesions, probably related to the thrombocytopenia the patient had. Gera and Georg[6] reported similar observations in MRI in their case. Both these patient were reported to have features of ADEM in the initial presentation with dengue infection. Our patient had thrombocytopenia at earlier presentation but her platelet count became normalized when she recovered from initial dengue infection. Again, she presented with seizures and encephalopathy after an interval of about three weeks from the initial febrile non neurological illness which is a characteristic feature in post infection ADEM. Most likely pathophysiology seems to be immune related secondary to dengue infection which is favored by this interval between initial dengue infection and encephalopathy and dramatic response to intravenous methyl prednisolone that led to both clinical, as well as radiological recovery.
Our patient did not have classical changes of ADEM in MRI and has lesions mainly involving cortical grey matter. We did not keep possibility of dengue encephalitis secondary to direct viral invasion in this patient as neurological manifestations were not present during initial acute febrile illness and rapid complete response to steroid therapy which is relatively unusual in patients with encephalitis. However, he can be diagnosed and labeled as encephalitis later due to immune related injury. There can be site restricted forms of ADEM such as pure encephalitis, myelitis, cerebellitis and optic neuritis. Marchioni et al.[9] found 20% patients of ADEM as pure encephalitis in their own series.
The natural history of ADEM in general is variable. Data from Asian countries have shown that the rate of progression to multiple sclerosis is low,[10] in contrast to Western population where the rate is higher due to unknown reasons.[11] Our patient had complete recovery; most of the adults in different series were left with residual degrees of disability after ADEM. Mortality rates of up to 20% have been reported which depend upon the level of consciousness on presentation, co-morbidities and extension of lesions. Children have better prognosis than adults.
No therapy has been established by controlled trials in ADEM. Use of high-dose steroids, plasma exchange, and intravenous immunoglobulin are based on the analogy of pathogenesis of ADEM with that of MS.
Conclusion
Dengue infection is endemic in developing countries posing a major public health problem. Clinical manifestations form a broad spectrum. Increasing evidence of neurotropism and atypical presentations by dengue virus emphasizes that clinician be aware of such association. Early diagnosis and timely intervention can reverse this potentially fatal disease.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65:848-5.
- [Google Scholar]
- Acute disseminated encephalomyelitis following dengue fever. J Infect Chemother. 2002;8:175-7.
- [Google Scholar]
- Acute disseminating encephalomyelitis following dengue haemorrhagic fever. Neurol India. 2010;58:599-601.
- [Google Scholar]
- Acute disseminating encephalomyelitis with haemorrhage following dengue. Neurol India. 2010;58:595-6.
- [Google Scholar]
- Pathogenesis, diagnosis, and treatment of acute disseminated encephalomyelitis. Curr Opin Neurol. 1999;12:395-401.
- [Google Scholar]
- Post infectious inflammatory disorders: Subgroups based on prospective follow-up. Neurology. 2005;65:1057-65.
- [Google Scholar]
- Descriptive study of acute disseminated encephalomyelitis and evaluation of functional outcome predictors. J Postgrad Med. 2010;56:12-6.
- [Google Scholar]
- Acute disseminated encephalomyelitis: A follow-up study of 40 adult patients. Neurology. 2001;56:1313-8.
- [Google Scholar]






