Translate this page into:
Traumatic Cerrebral Fungus: Experience From an Institution in North East India
Address for correspondence: Dr. Binoy Kumar Singh, Paras Hospital, Gurgaon, Haryana, India. E-mail: drbinoysingh@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Traumatic brain fungus is manifestation of neglected head injury. Although rare it is not uncommon. The patients are usually intact with good Glasgow coma (GCS) score inspite of complex injuries and exposed brain parenchyma but morbidity and mortality is very high with time if no proper and timely management is offered. There is very less study on traumatic brain fungus with no defined management protocols. So an attempt was made to explain in details the surgical strategies and other management techniques in patients with traumatic brain fungus.
Aims:
To study and evaluate the pattern of causation, clinical presentations, modalities of management of traumatic brain fungus and outcome after treatment.
Methods:
All patients with fungus cerebri, admitted to our centre from January 2012 to December 2015 were studied prospectively. All the patients were examined clinically and triaged urgently for surgery. CT head was done in all patients to look for any brain parenchymal injury. All patients were managed surgically. Outcome was assessed as per the Glassgow Outcome Score.
Results:
Total 10 patients were included in the study. 8 were men and 2 women. The patients’ ages ranged from 3-48 years (mean 31.6 years). The interval between initial injury and protrusion ranged from 3 days to 6 days (mean 4.1 days). Mean GCS at the time of presentation was 13.2.60% of the patients (n = 6) sustained moderate head injury. (GCS-9-13). Size of the fungus ranged from 5cm×3cm to 8cm×10cm.
Conclusion:
Early and proper local wound treatment prevents fungus formation. Pre-emptive antibiotics, AEDs and cerebral decongestants are recommended. Loose water-tight duroplasty prevents CSF leak. But mortality and morbidity can be reduced significantly if brain fungus is managed properly by applying basic surgical principles and antibiotic protocols combined with newer surgical modalities.
Keywords
External ventricular drain
Glasgow coma score
Glasgow outcome score
meningitis
traumatic brain fungus
INTRODUCTION
The traumatic cerebral fungus is a manifestation of neglected compound head injuries. It is defined as a protrusion of brain tissue through a defect in its covering, that is, scalp, skull, and dura. It is also termed as cerebral fungus or fungus cerebri. The cerebral fungus should be differentiated from “cerebral hernia” where there is a herniation of brain tissue through a defect in dura and bone but covered by the scalp.[1] In cerebral hernia, there is no infection or structural alteration of the herniated tissue, whereas they are the hallmark features of brain fungus. Traumatic brain fungus is neglected or unattended complex brain injury with high mortality. It usually results when the primary wound is not dealt with adequate care, especially when the scalp is sutured without proper dural closure and wound debridement. It is an uncommon entity with very limited study and almost no literature after 1943. Although uncommon it is not infrequent, especially in countries where neurosurgical set up is restricted to cities and in war zone where providing specialized neurosurgical attention to the victim is not feasible and at the same time, transportation of such victim needs time because of difficult terrain.
The formation of brain fungus is variable. Magnant (1927) stated that formation of brain fungus is obvious in 8–10 days. The formation of brain fungus can be as early 3 days but may be delayed up to 2 weeks. During the early stage, the fungus consists of pale soft pulsating cerebral tissue. It is adhered by flakes of lymph and superficial sloughs at this stage giving its appearance of healthy granulation tissue and dense scar formation at the margin of the fungus.
Aims and objectives
To study and evaluate the pattern of causation, clinical presentations, modalities of management and outcome of traumatic cerebral fungus.
MATERIALS AND METHODS
All patients with fungus cerebri, admitted in Gauhati Medical College and Hospital from January 2012 to December 2015 were studied prospectively as approved by the ethical committee of Gauhati Medical College and hospital. The patients were managed as per revised guidelines of management of penetrating brain injury.[23]
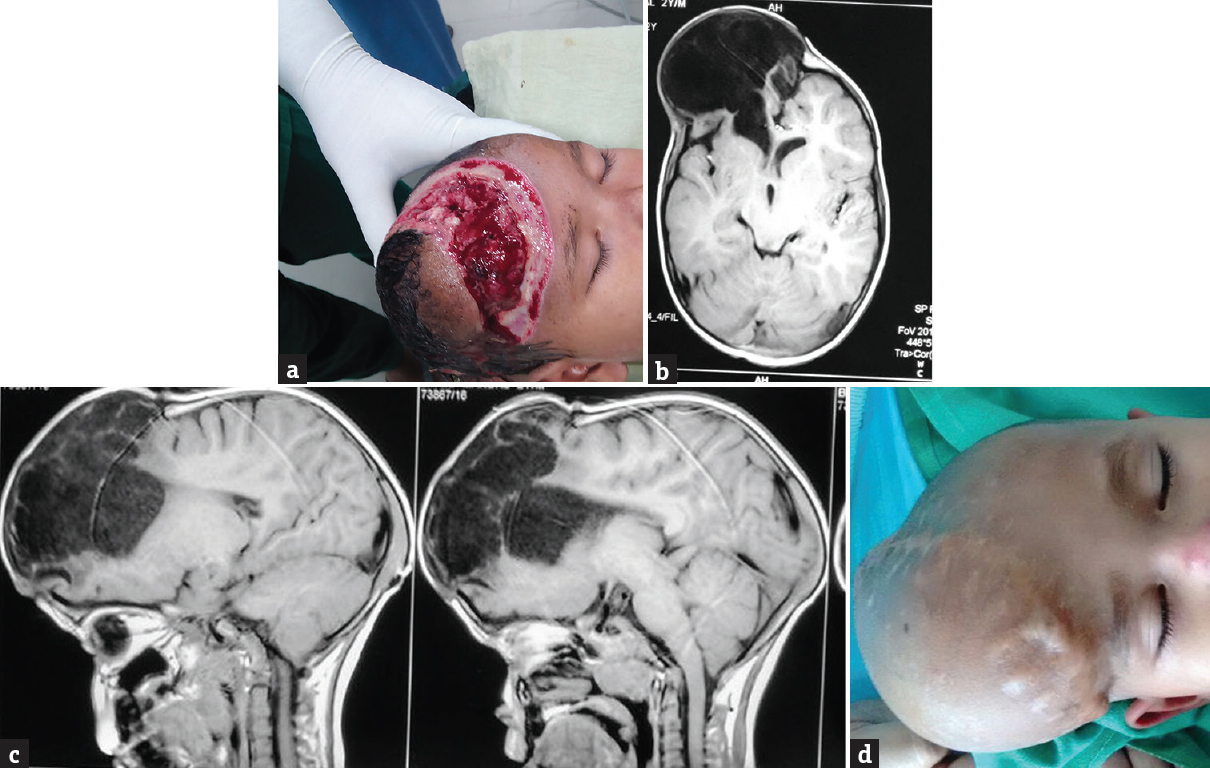
All the patients were examined clinically in the emergency department of our hospital as per trauma protocol and triaged urgently for surgery. Glasgow coma score (GCS) was noted. All patients with brain fungus were imaged emergently with unenhanced computed tomography (CT) scan regardless of exposed brain and fractures evident on clinical examination. In addition to the standard axial views with bone and soft tissue windows, coronal and sagittal sections were also considered. Three-dimensional (3D) reconstruction was done to have a better preoperative idea of the extent of the bony injury and proper planning of incision and exposure [Figure 1]. CT angiography was done in 1 patient with penetrating injury with intracerebral hematoma in the anterior cerebral artery-Acom junction region. Postoperatively, CT scan was done in all cases which were helpful in evaluating the development of intracranial hematomas, the presence of residual foreign body or bone fragments, and the extent of cerebral edema.
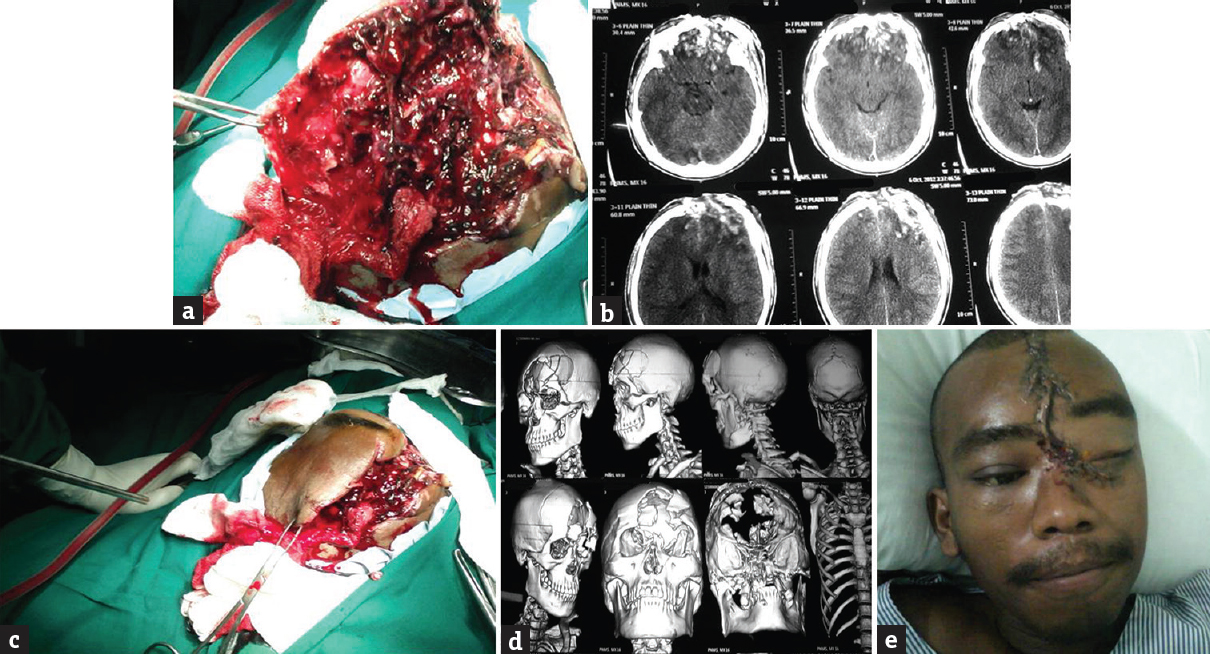
- (a) Herniated necrotic brain, (b) nonenhanced scan, (c) intra operative image showing d extent of injury, (d) three-dimensional computed tomography scan showing extent of bony injury, (e) postoperative image
All patients were managed surgically. Postoperative monitoring was carried out in the Neurosurgery Intensive Care Unit (ICU). All postoperative complications were recorded and managed. The outcome was assessed as per the Glasgow outcome score (GOS). All the patients were followed up as per GOS and any neurological deficits if the present were documented.
RESULTS AND OBSERVATIONS
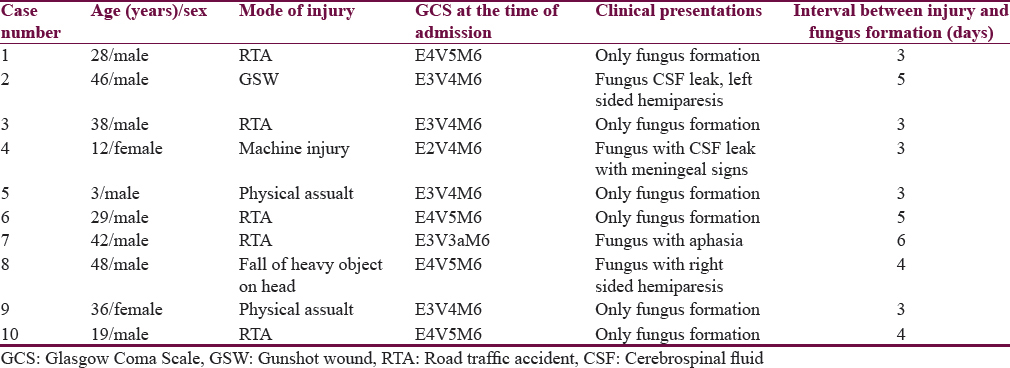
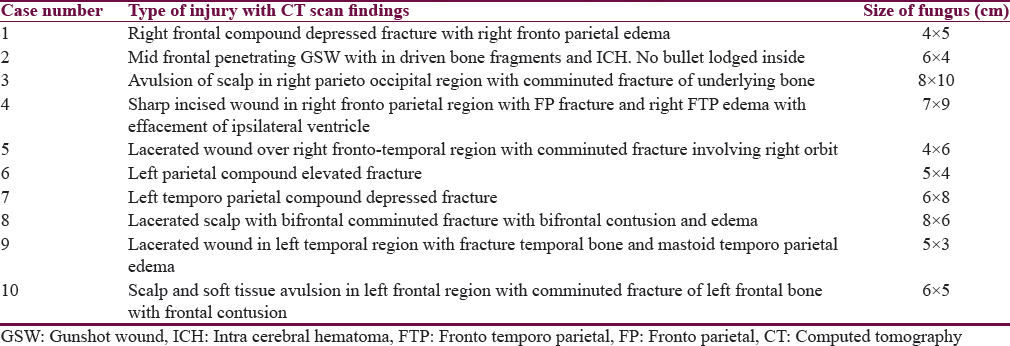
Totally 10 patients were included in the study. Eight were men and 2 were women. The patient's ages were from 3 to 48 years (mean 31.6 years). The interval between initial injury and protrusion ranged from 3 to 6 days (mean 4.1 days). Mean GCS at the time of presentation was 13.8.50% of the patients (n = 6) sustained a moderate head injury (GCS-9-13). The size of the fungus ranged from 5 cm × 3 cm to 8 cm × 10 cm. The fungus appeared like pale, soft pulsating brain tissue with superficial slough. The brain was soft and necrotic in two patients. Mode or pattern of injury included road traffic accident (RTA) (n = 5), physical assault (n = 2), fall of heavy object on the head (n = 1), and machine injury (n = 1) as shown in Table 1. Type of injury in all the patients included compound fractures with breach of underlying dura was noted. Injury details with respect to CT scan findings are depicted in Table 2. Focal neurological deficits were present in 3 patients in the form of right-sided hemiparesis, left-sided hemiparesis and aphasia, respectively.
Local treatment
All wounds, after proper inspection, were gently irrigated with normal saline. The wound was then covered with sterile paraffin gauze over which cotton gauze piece was applied. Care was taken not to manipulate the wound any further in the emergency room. Samples were collected immediately and sent for culture and sensitivity reporting.
Prophylactic antibiotics were administered to all patients, which included ceftriaxone, cefuroxime or amoxicillin and potassium clavulanate plus vancomycin[45] with metronidazole (antibiotics changed later as per culture and sensitivity report). Penicillin G was used in three patients (75,000–100,000 units/kg/dose IV 6 hourly). The prophylactic anti-epileptic cover was given in the form of sodium valproate to all cases. Antitetanus immunoglobulin was also given in all adult patients.
Anesthesia and surgery
All patients were operated microscopically under general anesthesia with maximum relaxants applying basic surgical principle as shown in Table 3. Patients were positioned in horseshoe with a proper antiseptic dressing of scalp and thigh to harvest tensor fascia lata for dural graft. Muscle fascia and pericranium were avoided for being infected and destroyed by primary injury. None of the patients was allowed to come out of effects of relaxants during operation. Patients were slowly and gradually extubated in the post-anesthetic care unit. The incision was made to incorporate the area that needed debridement and to preserve the vascular supply of the flap.
The margin of fungus is usually adherent to the granulation tissue covering the scalp and skull margin, especially in patients that present lately after fungus formation. Craniectomy was done, so that dural edges are clearly delineated. Diluted hydrogen peroxide was used in only grossly contaminated wounds. Peroxide was immediately removed by irrigating with normal saline Exposed brain parenchyma should be covered with moist saline large size cottonoid. Careful separation of these soft adhesions by pointed bayonet forceps, moist micro cottonoid, low power bipolar, micro scissors, and micro dissector which allowed of a complete reduction of the fungus. Dissection and manipulation were carried out with the outmost care and lots of patience so that the meningeal spaces are not laid open, and the cortex be not further injured. At a later stage, when healthy granulation tissue covers the surface, epithelialization of the area from its margins was rapid, and no graft was needed. In a very extensive loss of superficial tissue, it might be that a Thiersch graft would be the only mode of management in such patients. None of our patients needed Thiersch graft.
All necrotic brain tissue, bone fragments, blood clot, and foreign bodies were removed. Gentle debridement of devitalized brain was performed using a combination of low power suction with blunt tips, bipolar cautery, and irrigation. Cortical surface should be gently irrigated and cleaned of micro-clots before closure. The ultrasonic surgical aspirator was used for corticectomy in fungus with extensive necrosis as shown in Figure 2. Significant mass effect with cerebral edema was noticed in one patient in whom a decompressive hemicraniectomy plus lax duraplasty with tensor fascia lata homograft was done. Limited corticectomy had to be done in two patients with extensive fungation. The primary orbital repair was done in one patient along traumatic brain fungus. Herniated brain was preserved as much as possible to have a better functional outcome. The necrotic and lacerated muscle were extensively debrided. Small bony chips and contaminated bones are discarded and only healthy non contaminated bone is replaced back in one patient.
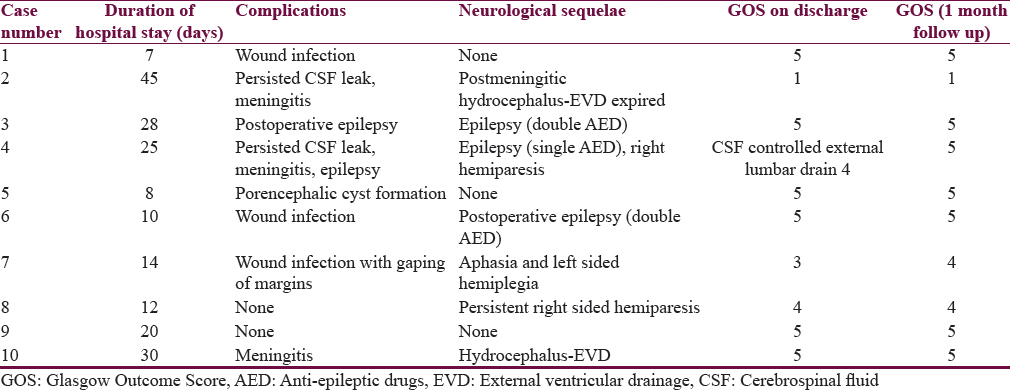
- (a) Brain fungus, (b) magnetic resonance imaging axial cut showing porencephalic cyst, (c) magnetic resonance imaging sagittal cut, (d) postoperative porencephalic cyst
Closure
Loose duraplasty was done in all cases.[6] Tensor fascia lata was used in all cases, but artificial dura was used in a single patient. Dura closed water tight with 4-0 PDS. Galea was closed with interrupted sutures 2-0/3-0 Polyglactin. Edges of the wounds were trimmed gently for fresh margins. The skin was closed using 2-0/3-0 nylon simple interrupted sutures. Closed drainage was placed in all cases. split skin grafting (SSG) was required in one patient with skin loss due to necrosis and debridement.
External ventricular drain placement
In two patients, external ventricular drain (EVD) was placed as they developed meningitis with hydrocephalus. It was done in odds ratio with all aseptic and antiseptic measure with subcutaneous tunneling. It was also used as a route to administer intrathecal vancomycin. EVD was left for 7 days.
External lumbar drain
EVD was placed in one patient at L4–L5 space under local anesthesia aseptically bedside in Neuro ICU.
Cranioplasty
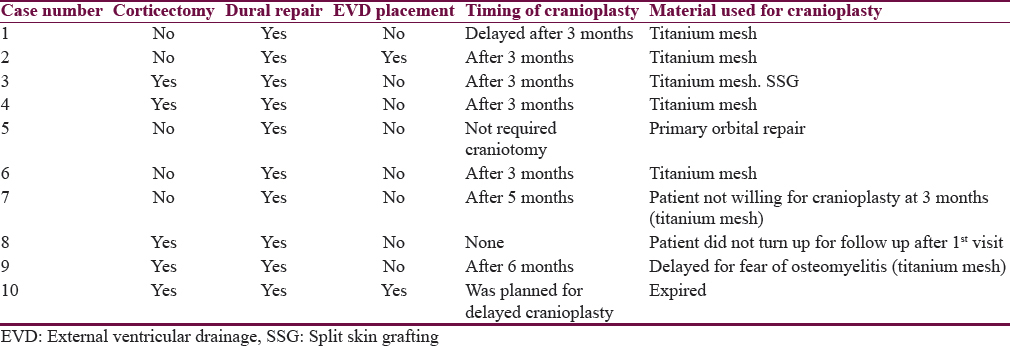
Bone was replaced immediately in one patient. Cranioplasty was done after 3 months in 5 patients. Cranioplasty was delayed (6 months) in one patient due to fear of osteomyelitis. Cranioplasty was done after 5 months in one patient as he was not willing for cranioplasty at 3 months. One patient did not turn up for follow-up. The material used for cranioplasty was titanium mesh (7 patients) and bone cement (1 patient) as illustrated in Table 3.
Complications
Postoperative complications included persisted postoperative cerebrospinal fluid (CSF) leak (n = 2), meningitis (n = 3), wound infection (n = 3), and epilepsy (n = 2). Wound infection was treated with regular dressing with povidone–iodine solution and debridement. Two patients needed secondary suturing. One patient (Case no 4) required SSG at later date when there was the growth of healthy granulation tissue. In spite of prophylactic anti-epileptic medications, the seizure was controlled with two drugs by adding levetiracetam in two patients as the developed postoperative seizure. CSF leak was associated with post meningitis hydrocephalus in one patient for which EVD was placed, and intrathecal antibiotics were given. Postoperative CSF leak was controlled in one patient with acetazolamide and external lumbar drain. One patient developed porencephalic cyst formation [Figure 2].
One patient died (mortality-10%) due to refractory meningitis with hydrocephalus; although, he was managed aggressively with EVD and intrathecal antibiotics.
The duration of hospital stay ranged from 7 to 45 days (mean-19.9 days).
At follow-up, 7 patients had the good functional outcome (GOS-4 in 3 patients and 5 in 4 patients) and 2 had a poor outcome (GOS-2). Neurological sequelae at follow-up were epilepsy (n = 3), left-sided hemiparesis (n = 1), right-sided hemiparesis (n = 1), and aphasia (n = 1) [Table 4].
DISCUSSION
Traumatic brain fungus is a serious complication of head injury and is very difficult to manage if associated with CSF leak and meningitis. This complication of head injury is almost always preventable if proper and early management of compound head injury is done.
The literature on traumatic brain fungus is very limited. The only published series on traumatic cerebral fungus was way back in 1943 by O’Connell.[1] The study included 11 patients, all soldiers wounded in the war.
In their series, all the patients were male soldiers, whereas in our study, there were 8 men and 2 women. Ages in their study ranged from 11 to 59 years with an average of 36.97 years. In our study, patients ages were 3–48 years (mean 31.6 years).
The most common mode of injury was gunshot wounds (n = 6), followed by injury by bomb fragments (n = 3), getting buried under bombed building (n = 2), and injury due to a collision at sea (n = 1). In our series, the modes of injury were RTA (n = 3), gunshot wound (n = 2), bomb blast (n = 1), physical assault (n = 2), machine injury (n = 1), and fall of heavy object on head (n = 1).
The size of fungus in O’Connell[1] ranged from 1 cm × 2 cm to 9 cm × 2.5 cm. In our study, it ranged from 5 cm × 3 cm to 8 cm × 10 cm. Eight of eleven patients in their series presented with focal neurological deficits in the form of hemiparesis, hemianesthesia and aphasia, whereas 3 out of 10 patients in our series had focal neurological deficits on presentation in the form of right-sided hemiparesis, left-sided hemiparesis, and aphasia.
The investigations done in their series were limited as it was pre-CT scan era. They resorted to skull X-rays and CSF analysis. All our patients underwent CT head (plain and contrast) with 3D reconstruction and cerebral CT angiography in one patient.
Treatment in their study comprised lowering intracranial pressure by lumbar drainage in all patients, with EVD placed in one patient. Local treatment in the form of dressing with antibiotic soaked gauzes. Paraffin gauze and sequential firmly applied bandages over the fungus were used for reduction of the fungus. A skin graft was applied over the fungus in 1 case which was accepted nicely.
In our study, early and less aggressive surgical management was undertaken. Thorough debridement, wound toileting, removal of clots, necrotic debris, and foreign body was done using suction, bipolar cautery, and irrigation. Careful, meticulous, limited corticectomy had to be done in 2 cases. EVD was placed in 2 cases as they presented with meningitis with hydrocephalus which was also used to deliver intrathecal vancomycin. Dura was repaired in all cases with loose duraplasty using tensor fascia lata in all cases but one in which artificial dura was used.[3] The only craniectomy also known as Leriche's (1916) operation of enlarging trepanation which was done in earlier times to help reduce fungus is founded on an incorrect conception of the etiology of a cerebral fungus. It is the margins of the dural defect which form the orifice through which fungation occurs. The removal of the skull from around its margins, leaving the dura intact, is believed to do nothing to relieve a hypothetical venous congestion in the fungus. Further, it has been suggested above that venous congestion due to strangulation is not a factor of importance in the etiology of the condition.
EVD was done in their study for fungus reduction, but we did decompressive craniectomy with lax duraplasty to accommodate the edematous brain. EVD was done in our study only in case of postmeningitic hydrocephalus.
Cranioplasty was done in 5 patients after 3 months, and in two patients, it was done after 5 months and 6 months, respectively. Intravenous antibiotics in the form of sulfonamides were given in each case in their study. Ceftriaxone or amoxicillin and potassium clavulanate with vancomycin, Penicillin G and metronidazole was started in all patients in our series and changed later as per culture and sensitivity report.[45] All patients were given anti-epileptic cover in the form of sodium valproate. Two of the patients in their series developed meningitis which were aggressively managed with intrathecal antibiotics instilled by EVD catheter, but one patient did not recover and succumbed to it.[6] None of the patients developed brain abscesses.
In this study, postoperative complications included CSF leak (n = 3), meningitis (n = 4), wound infection (n = 2), and epilepsy (n = 3). There were no mortalities in their study. One patient died (mortality-10%) due to refractory meningitis in ours.
At follow-up, in their study 9 out of 11 patients had neurological sequelae in the form of epilepsy, persistent hemiparesis, and dysphasia. In this study, 7 patients had the good functional outcome (GOS-4 in 2 patients and 5 in 5 patients), and 2 had a poor outcome (GOS-2). Neurological sequelae at follow-up were epilepsy (n = 3), left-sided hemiparesis (n = 1), right-sided hemiparesis (n = 1), and aphasia (n = 1).
CONCLUSION
Management of traumatic brain fungus requires basic surgical and technical skills. Early and proper local wound treatment prevents fungus formation. Preemptive antibiotics, AEDs and cerebral decongestants are recommended. Penicillin G can still be considered in compound head injury if not allergic with good results. The ultrasonic aspirator can be considered as a better modality in compare to suction devices for corticectomy in patients of brain fungus with a necrotic brain. Loose water-tight duraplasty prevents CSF leak. Homograft (fascia lata) is preferred over artificial dura. The bone must be preserved as much as possible and replaced only if no contamination is suspected. Traumatic brain fungus is almost forgotten because it is an infrequent entity, but when it presents, morbidity and mortality is very high in spite of good GCS score on admission if not managed properly. Prognosis is worst if traumatic brain fungus was associated with CSF leak. Traumatic brain fungus is uncommon but not history, in fact, it needs to be revised management protocols to decrease the morbidity and mortality associated with it. Urgent triage of these patients in the emergency department and immediate intervention play a pivotal role in avoidance of the dreaded complications which invariably ensue, if unattended.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Traumatic cerebral fungus. British Journal of Surgery. 1943;30:201-12. [//dx.doi.org/10.1002/bjs.18003011905]
- Part 1: Guidelines for the management of penetrating brain injury. Introduction and methodology. J Trauma. 2001;51(2 Suppl):S3-6.
- [Google Scholar]
- Surgical management of penetrating brain injury. J Trauma. 2001;51(2 Suppl):S16-25.
- [Google Scholar]
- Use of antibiotics in penetrating craniocerebral injuries.“Infection in Neurosurgery” Working Party of British Society for Antimicrobial Chemotherapy. Lancet. 2000;355:1813-7.
- [Google Scholar]
- Antibiotic prophylaxis for penetrating brain injury. J Trauma. 2001;51(2 Suppl):S34-40.
- [Google Scholar]






