Translate this page into:
Thoracic hypertrophied ligamentum flavum: A retrospective study of pre-operative factors and surgical outcome
*Corresponding author: Pawan Kumar Verma, Department of Neurosurgery, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India. dr.pawankverma@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ranjan N, Shukla A, Verma PK, Kanjilal S, Kumar A, Mehrotra A, et al. Thoracic hypertrophied ligamentum flavum: A retrospective study of pre-operative factors and surgical outcome. J Neurosci Rural Pract. 2024;15:573-9. doi: 10.25259/JNRP_240_2024
Abstract
Objectives:
Thoracic hypertrophied ligamentum flavum (HLF) is a rare cause of thoracic myelopathy which requires surgical treatment. The study aims to analyze the pre-operative factors and surgical outcomes in patients with thoracic myelopathy secondary to HLF.
Materials and Methods:
This is a retrospective study of patients who underwent surgery for thoracic myelopathy due to HLF at a single center from December 2015 to November 2023. We included patient’s demographic data, clinical symptoms, radiological details, operative details, and outcome. We used Nurick’s grading system for outcome evaluation in pre-operative, post-operative, and follow-up. Relationship of various pre-operative parameters was evaluated with surgical outcomes on binary scale (favorable group [Nurick’s grade 1 and 2] and unfavorable outcome [Nurick’s grade 3–5]) using univariate and multivariate analysis.
Results:
A total of 57 patients were included in the study. On analyzing various prognostic factors with respect to favorable and unfavorable outcomes using univariate analysis, four factors came out to be statistically significant which were segments involved (multi vs. single segment involvement) (P < 0.05), walking difficulty (P < 0.05), intramedullary signal changes on T2-weighted magnetic resonance imaging (MRI) (P < 0.001), and pre-operative Nurick’s grade (P < 0.001). On multivariate analysis, only one factor, pre-operative Nurick’s grade came out statistically significant.
Conclusion:
Various factors are important in predicting the outcome of a patient with thoracic myelopathy secondary to HLF. The most important of which is pre-operative Nurick’s grade. Other factors that also affect the outcome are the presence of multisegmented disease and intramedullary T2 signal changes on MRI.
Keywords
Laminectomy
Cerebrospinal fluid leak
Nurick grade
Myelopathy
Hypertrophied ligamentum flavum
INTRODUCTION
Ligamentum flavum is a broad paired spinal ligament bridging the adjacent laminae of the vertebral column. It extends from C-2 to S-1.[1-3] Ligamentum flavum can undergo hypertrophy, calcification, or ossification.[4] Hypertrophied ligamentum flavum (HLF) is a result of degenerative spine disease which was first described by Polgar in 1920.[5] This occurs through endochondral ossification of hypertrophied fibrous tissue within the ligaments. HLF is often reported as endemic to Japan but reports from other areas of the world have increased. However, 88.8% of reports on HLF in literature come from Japan with Caucasian being the second most reported group with 8.2% reports in literature.[2] The most common location of HLF in the spine is the lower thoracic region with T9–T12 being the most affected.[6-8] This may be due to it being the transition zone with increased motion and microtrauma to ligamentum flavum.[6] HLF can present in a multitude of ways ranging from pain and paresthesia to spastic paraparesis with or without sensory and autonomic symptoms. The most used method of treatment is posterior decompression by laminectomy; however, laminoplasty has been described as an alternative because of the late neurological deterioration and pain due to post-laminectomy kyphosis.[4] In this retrospective analysis, we try to study the key factors, which are associated with the outcome of the patient with HLF who underwent laminectomy and decompression at a single institute. The study of these factors is important for an informed surgical decision and prognosis of the patients.
MATERIALS AND METHODS
Study participants
This is a retrospective study of patients who underwent spinal surgery for HLF at a single center from December 2015 to November 2023.
Inclusion criteria
Patients with a diagnosis of thoracic myelopathy secondary to HLF were included in the study.
Exclusion criteria
Patients who suffer myelopathy due to other spinal diseases, including cervical or lumbar HLF, infection, tumor, spinal deformity, fracture, history of previous spinal surgery, and follow-up <6 months were excluded from the study. Patients who had incomplete clinical and imaging data and patients with other pathologies causing thoracic myelopathy including ossification of posterior longitudinal ligament were excluded.
Study parameters
The clinical and demographic data, clinical symptoms, comorbidities, radiological details, operative details, and outcomes were collected from the Hospital Information system. The variables included are age, sex, duration of symptoms, presenting symptoms such as back pain, walking difficulty, limb paresthesia, bladder-bowel involvement, associated prolapsed intervertebral disc (PIVD), duration of hospital stay, site of HLF, number of segments involved, T2 hyperintensity changes on magnetic resonance imaging (MRI), intra-operative dural tear, cerebrospinal fluid (CSF) leak, and wound complication. All patients were followed up by outpatient department visits or through telephonic conversations.
Surgical procedure
Indication of surgical treatment is symptomatic HLF. The main goal of surgical treatment was the decompression of the complete cord as well as the nerve root at the involved level. A high-speed cutting drill was used to perform laminectomy while thinning of the ossified ligamentum was done using a diamond bit. Lateral drilling of the lamina was performed till the exiting roots along with the dural sheaths were made bare. The use of Kerrison’s rongeurs and bone nibbler was kept to a minimum, and only a 1-mm rongeur was used for peeling off papery thin ligamentum flavum at the end of drilling. The rongeur negotiation was done through the lateralmost part of the drilled lamina assuming the lateral limit of the thecal sac. Decompression was continued till the venous bleeding was noted from the lateral gutter, which suggested adequate decompression [Figure 1]. In case of a CSF leak noted during surgery, primary repair of the dura was done whenever it was possible. Artificial dural substitutes were used in cases where primary repair was not possible.
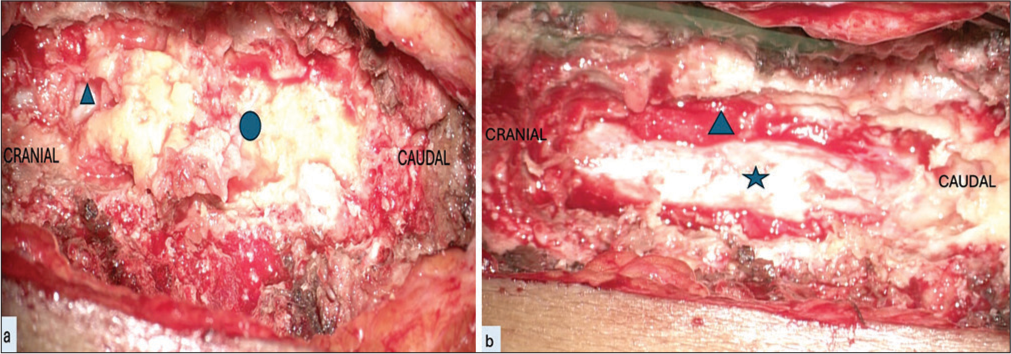
- Intraoperative images of dorsal hypertrophied ligamentum flavum (HLF) surgery done using laminectomy and excision of HLF. (a) Intraoperative image showing dorsal HLF (shown in blue circle) after laminectomy which is done using combination of cutting and diamond drill. Laminectomy is done till normal dura is visualized (shown in blue triangle). (b) Intraoperative image showing dura (shown in blue star) after excision of HLF. Laminectomy should be done till lateral gutters are visualized. Visualization of lateral gutter is generally characterized by bleeding from venous plexus which is controlled with gelfoam (shown in blue triangle).
Outcome
The outcome was evaluated using Nurick’s grading system[9] in pre-operative, post-operative, and follow-up. The outcome after surgery was compared on a binary scale by grouping it into favorable group (Nurick’s grade 1 and 2) and unfavorable outcome (Nurick’s grade 3–5). The binary scale is also used for age (>50 years and <50 years), duration of symptoms (<6 months and >6 months), and number of segments involved (up to 2 segments and >2 segments) to see the relationship of these parameters with outcome. The other determinants studied against the outcome groups were pre-operative Nurick’s grade, walking difficulty, limb paresthesia, bladder-bowel involvement, associated PIVD, duration of hospital stay, site of HLF, and T2 hyperintensity changes on MRI and CSF leak.
Statistical analysis
This was done in IBM Statistical Software for the Social Sciences Statistics version 23.00 Armonk, New York, USA. The Shapiro–Wilk test was used to assess the normality of the data. The continuous variables were expressed as mean ± standard deviation and median values (1st quartile– 3rd quartile), while categorical variables were expressed with frequency and associated percentages. Logistic regression analyses for predictors of unfavorable outcomes were performed using the Mantel–Cox test and backward Wald logistic regression and characterized by odds ratios, 95% confidence intervals (CIs), and the Wald test p values. Logistic regression models included patients’ age group (<50 years = reference [ref]) and sex (male = reference [ref]), number of segments involved (<2 [ref] vs. >2), intramedullary signal changes (no [ref] vs. yes), pre-operative Nurick grade (<2 [ref] vs. >2), and walking difficulty (no = reference). The statistical significance was set to a P < 0.05.
RESULTS
Patient characteristics
A total of 57 patients of thoracic myelopathy secondary to thoracic HLF were included in the study. There were 36 male (63.2%) and 21 female (36.8%) patients who ranged in age from 26 years to 79 years (mean age – 50.9 years). The mean duration of symptoms was 15.6 months before treatment. The most common presenting symptoms included backache (70.2%), paresthesia (82.5%), walking difficulty (78.9%), and urinary disturbances (38.8%). Spasticity and lower-limb weakness were present in all the patients. The patients’ symptoms are summarized in a bar chart [Figure 2]. 17 patients had pre-operative Nurick’s grade of 1 or 2 (29.8%) and 40 patients had pre-operative Nurick’s grade 3 or greater (70.2%). The average hospital stay of patients was 7 days (range 3–24 days) and the mean follow-up of 10.5 months. 21 patients (36.8%) had single-level involvement whereas 36 patients (63.2%) had multilevel involvement. Only two patients (3.5%) had upper dorsal involvement, 9 patients (15.8%) had mid-dorsal level involvement whereas the majority of the patients, i.e., 46 patients (80.7%) had lower dorsal involvement. We also saw the presence of associated PIVD in 30 patients (52.6%) of which only four patients required discectomy along with laminectomy. Cord signal hyperintensities on T2 imaging were present in 32 (56.1%) patients pre-operatively [Figure 3].
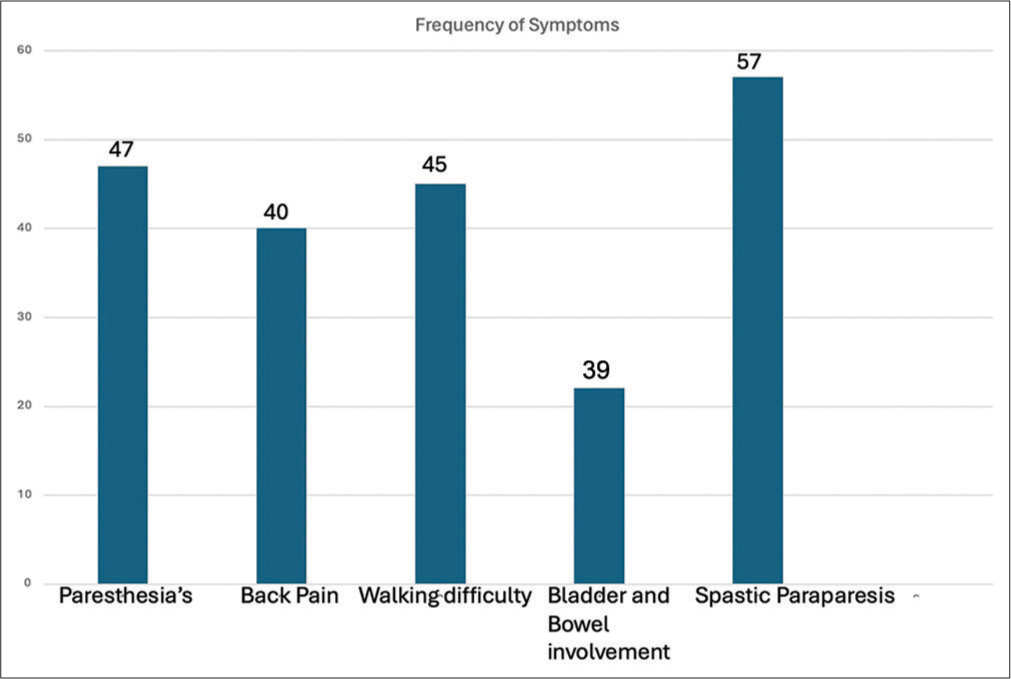
- Bar chart representation of symptoms.
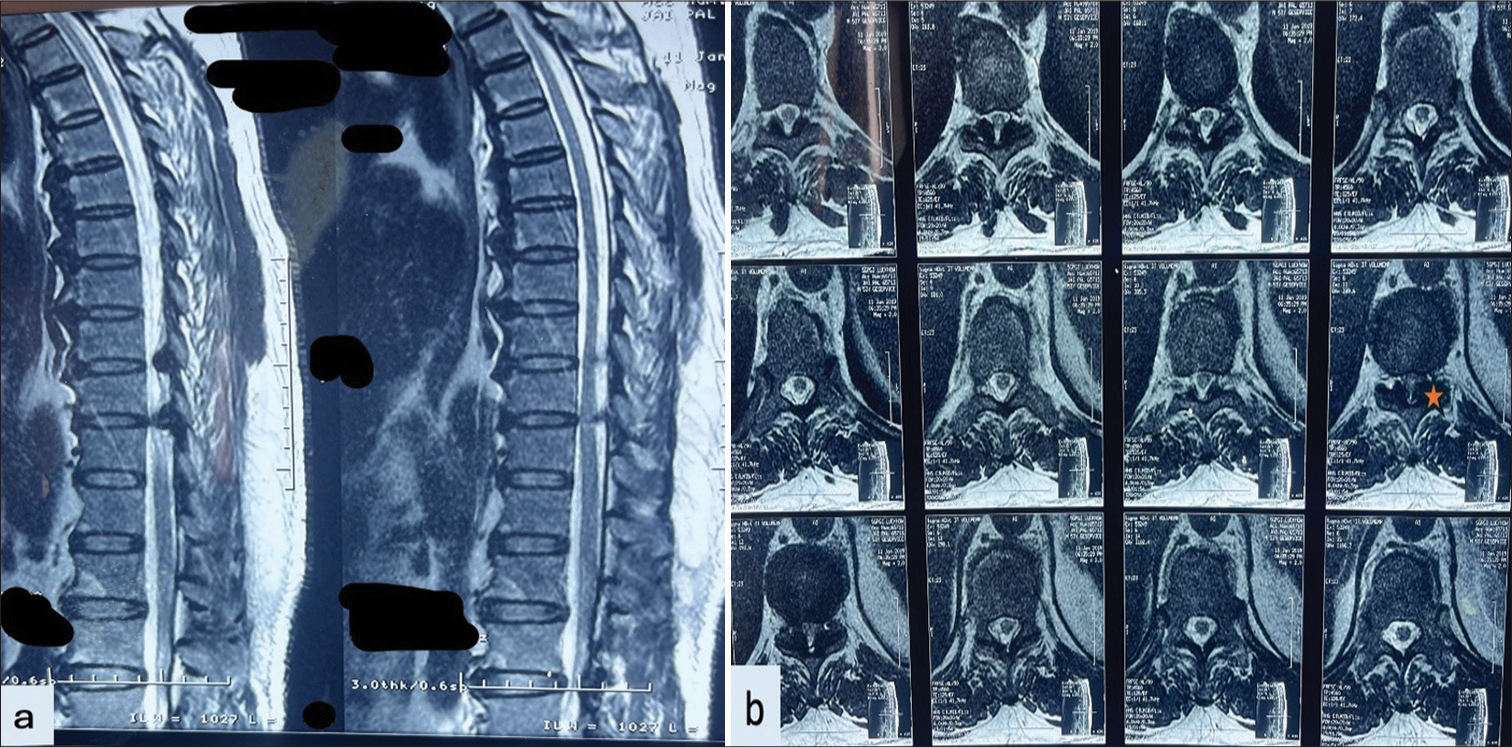
- Magnetic resonance imaging (MRI) of patient with dorsal hypertrophied ligamentum flavum (a) MRI sagittal T2-weighted image shows a beak-like projection of hypertrophied ossification of the ligamentum flavum (OLF) causing compression over the cord with associated cord signal changes. (b) MRI axial T2-weighted image showing compression of dorsal hypertrophied OLF (shown in star).
Intra-operative details
All patients underwent surgical decompression through laminectomy and excision of HLF. The most common procedure performed was D9–D10 laminectomy and D9– D11 laminectomy. In addition, four of our patients required discectomy along with laminectomy. Intra-operatively, 19 (33.3%) had CSF leak in the form of dural tear. In 13 patients, the ligamentum flavum could not be excised completely and it was thinned out by careful drilling and floated by making lateral gutters.
Complications
Post-operative CSF leak was seen in 7 of our patients (12.3%) and 3 patients (5.2%) had localized infection which was managed conservatively. 12 cases (21%) deteriorated neurologically in the immediate post-operative period, 20 patients (35%) had shown improvement in power, and in 25 patients (44%), power remained the same. None of the patients required second surgery for ossification at another level during follow-up. The clinical, radiological, intraoperative, post-operative details, and complications are summarized in Table 1.
| Characteristics | Variable | Value |
|---|---|---|
| Male | 36 (63.2%) | |
| Female | 21 (36.8%) | |
| Age | <50 years | 28 (49.1%) |
| >50 years | 29 (50.1%) | |
| Duration of symptoms | < 6 months | 28 (49.1%) |
| >6 months | 29 (50.9%) | |
| Clinical Features | Paresthesia | 47 (82.5%) |
| Back pain | 40 (70.2%) | |
| Walking Difficulty | 45 (78.9%) | |
| Bladder and bowel involvement | 22 (38.6%) | |
| Pre-operative Nurick Grade | Grade 0 | 1 (1.8%) |
| Grade 1 | 7 (12.3%) | |
| Grade 2 | 9 (15.8%) | |
| Grade 3 | 16 (28.1%) | |
| Grade 4 | 7 (12.3%) | |
| Grade 5 | 17 (29.8%) | |
| Segment involvement | Single | 21 (36.8%) |
| Multiple | 36 (63.2%) | |
| Site | Upper dorsal | 2 (3.5%) |
| Mid dorsal | 9 (15.8%) | |
| Lower dorsal | 46 (80.7%) | |
| Associated PIVD | Present | 30 (52.6%) |
| Absent | 27 (47.4%) | |
| IM sensitivity changes | Present | 39 (68.4%) |
| Absent | 18 (31.6%) | |
| Post-op Nurick grade | Grade 0 | 3 (5.3%) |
| Grade 1 | 7 (12.3%) | |
| Grade 2 | 7 (12.3%) | |
| Grade 3 | 10 (17.5%) | |
| Grade 4 | 8 (14.0%) | |
| Grade 5 | 22 (38.6%) | |
| Neurological improvement | Yes | 14 (24.6%) |
| No | 43 (75.4%) | |
| Dural Tear | Yes | 19 (33.3%) |
| No | 38 (66.7%) | |
| Post op CSF leak | Yes | 7 (12.3%) |
| No | 50 (87.7%) |
PIVD: Prolapsed intervertebral disc, CSF: Cerebrospinal fluid, IM: Intramedullary.
Prognostic factors
On analyzing the various pre-operative factors mentioned under the materials and method section, it was found that pre-operative Nurick’s grade (P < 0.001, 95% CI [36.742–10597]), walking difficulty (P < 0.05, degree of freedom – 1, 95% CI [1.276–18.821]), multiple segment involvement (>2) (P < 0.05, degree of freedom 1, 95% CI [1.514–37.159]), and intramedullary signal changes on T2-weighted (T2W) image MRI (P < 0.001, degree of freedom – 1, 95% CI [3.501–52.833]) have reached the statistically significant P-value [Table 2]. Further using univariate analysis, all four factors were found significant. However, when multivariate analysis was done, only pre-operative Nurick’s grade came out to be significant.
| Parameters | Favourable outcome n=17 (29.8%) | Unfavourable outcome n=40 (70.2%) | P-value |
|---|---|---|---|
| Age | |||
| <50 years | 9 (15.8%) | 19 (33.3%) | 0.707 |
| >50 years | 8 (14.0%) | 21 (36.8%) | |
| Duration of symptoms | |||
| <6 months | 9 (15.8%) | 19 (33.3%) | 0.707 |
| >6 months | 8 (14.0%) | 21 (36.8%) | |
| Paresthesia | |||
| Yes | 14 (24.5%) | 33 (57.9%) | 1.000 |
| No | 3 (5.3%) | 7 (12.3%) | |
| Back Pain | |||
| Yes | 14 (24.5%) | 26 (45.6%) | 0.190 |
| No | 3 (5.3%) | 14 (24.6%) | |
| Walking difficulty | |||
| Yes | 10 (17.5%) | 35 (61.4%) | 0.029* |
| No | 7 (12.3%) | 5 (12.5%) | |
| Bladder and bowel involvement | |||
| Yes | 6 (10.5%) | 16 (28.1%) | 0.738 |
| No | 11 (19.3%) | 24 (42.1%) | |
| Segments involved | |||
| Single | 15 (26.3%) | 20 (35.1%) | 0.007* |
| Multiple | 2 (3.5%) | 20 (35.1%) | |
| IM signal changes | |||
| Yes | 5 (8.7%) | 34 (59.6%) | <0.001* |
| No | 12 (21.1%) | 6 (10.5%) | |
| Pre – op Nurick Grade | |||
| Good | 16 (28.1%) | 1 (1.8%) | <0.001* |
| Poor | 1 (1.7%) | 39 (68.4%) | |
| Associated PIVD | |||
| Present | 9 (15.8%) | 21 (36.8%) | 0.976 |
| Absent | 8 (14.0%) | 19 (33.3%) |
DISCUSSION
Etiopathogenesis
HLF which was previously seen as endemic to Japan is now being reported from various parts of the world including the Indian subcontinent.[7,10,11] However, it is still a relatively rare entity. Ligamentum flavum is a bridge connecting the lamina posteriorly and has two portions, the medial interlaminar portion and the lateral capsular portion.[11] The capsular part of the ligamentum flavum is ossified early followed by ossification of the medial portion which compresses the posterior portion of the spinal cord. This process further proceeds in a craniocaudal direction along the posterior margin of the spinal canal in a linear fashion which looks like a beak-like outgrowth.[10] The most common location of thoracic HLF is lower thoracic vertebrae (T9–T12).[6,7,8] This is due to lower thoracic vertebrae transitioning to lumbar vertebrae which causes greater mobility leading to frequent mechanical stress. Furthermore, the unique orientation of zygapophyseal joints which facilitates greater rotatory motion leads to further instability.[12] In our study, we found a similar trend with 46 cases (80.7%) involving the lower dorsal region. It is also important to note that patients with diffuse idiopathic skeletal hyperostosis,[13] fluorosis, diabetes, and ankylosing spondylosis have a higher incidence of HLF. In our study, two cases (3.5%) were associated with ankylosing spondylosis, and six cases (10.5%) were associated with diabetes.
Clinicoradiological presentation
The HLF generally presents as spastic paraparesis with or without sensory and autonomic impairment. HLF can cause posterior column dysfunction which causes loss of balance.[11,13] This along with spastic paraparesis further exacerbates already existing walking difficulty. In our study, we found spastic paraparesis in all the patients, paresthesia in 47(82.5%), back pain in 40 (70.2%), walking difficulty in 45 (78.9%), and bladder and bowel involvement in 22 (38.6%). Before the advent and widespread use of MRI for neurological diagnosis, myelography was considered the best diagnostic tool to detect HLF. Axial computed tomography (CT) scan can show the contour and density of the ossification changes in ligamentum flavum. Sato et al.[14] gave a CT classification of HLF into five types (lateral, extended, enlarged, fused, and tuberous). At present, MRI is the modality of choice for the diagnosis of HLF. MRI shows HLF as hypointense on T1 and T2 weighted;[15] however, intramedullary signal changes generally appear hyperintense on T2-weighted images, probably due to edema in gray or white matter, loss of nerve cells, Wallerian degeneration, gliosis, and demyelination.[16-18] In our study, intramedullary signal changes on MRI were present in 32 cases (56.1%). On univariate analysis, IM signal changes came out to be significant in relation to surgical outcomes. However, on multivariate analysis, it came out to be insignificant.
Management
Conservative treatment is not indicated in preventing disease progression and neurological deterioration. Surgical treatment is generally considered for symptomatic patients of HLF. The most common surgical procedure is posterior decompression of neural elements through laminectomy and complete resection of HLF. In our study, all cases underwent laminectomy and complete resection of HLF. Other techniques used for the treatment of HLF are laminoplasty,[4] foraminotomy,[19] image-guided laminotomy,[20] en bloc resection,[21] and laminectomy with fusion.[22]
Complication
One of the major intra-operative complications of HLF surgery is a dural tear. In our series, intraoperative dural tear was noted in 19 patients (33.33%). If any dural tear is noted in the intra-operative period, it should be repaired immediately. Post-operative CSF leak was seen in 7 of our patients (12.3%), and 3 patients (5.2%) had localized infections, which were managed conservatively. Neurological deterioration can occur immediately following surgery and is mainly due to unintended intra-operative spinal cord manipulation. This complication can be kept to a minimum if during surgery high-speed pneumatic drills followed by thin footplate rongeurs (1 mm) are used. In our series, we noted a post-operative deterioration in 11 of our patients (19.29%). Another dreaded complication of HLF surgery is epidural hematoma, which can cause spinal cord compression.[13] We did not encounter this complication in our study. Some authors have raised concerns that laminectomy can lead to kyphotic deformity and late-onset neurological deterioration or localized back pain.[13] We have not encountered any such complication in our follow-up.
Outcome and prognostic factors
In our study, 21 patients (36.8%) had improvement in power, 25 patients (43.8%) had the same power, and 11 patients (19.29%) had deterioration in power in post-operative follow-up. In our study, we found that pre-operative neurological status walking difficulty, multiple segment involvement (>2), and T2W intramedullary signal changes on MRI were found to be significant in determining good neurological outcomes on univariate analysis. When analyzed through multivariate analysis, only pre-operative neurological status came out to be significant. Posterior compression over the spinal cord by the HLF causes walking difficulty (because of spastic paraparesis plus posterior column involvement) due to chronic changes of gray matter (ischemic necrosis, neuronal loss, or chromatolysis) and white matter (demyelinating changes).[23] Significant chronic compression can lead to irreversible neuronal changes generally preceded by apoptosis.[24] This likely explains no improvement of weakness and persistence of residual spasticity at follow-up due to irreversible changes within the cord. However, the generalizability of prognostic factors will require a multicenter prospective study with a large sample size as our study is the experience of a single tertiary center.
CONCLUSION
HLF forms one of the rare causes of thoracic myelopathy. The pathogenesis of thoracic HLF is mainly due to localized mechanical stress on the ligament resulting in single and multiple-segment disease. Classical presentation of HLF is paraparesis associated with posterior column and bladder-bowel involvement. Wide laminectomy with excision of HLF is the only way to get rapid recovery of paraparesis and other symptoms. However, various factors such as poor pre-operative Nurick’s grade (most important), walking difficulty, multi-segment (>2) involvement, and T2W intramedullary hyperintensity changes are the important determinants of successful outcomes. These predictors may prove a valuable guide to predict the post-operative recovery in patients of thoracic myelopathy due to HLF.
Ethical approval
The study is in compliance with Helsinki Declaration of 1964, and since this is a retrospective study, approval by institutional board is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consents.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Gray's anatomy of the human body. (20th ed). Philadelphia, PA: Lea and Febiger; 1918.
- [CrossRef] [Google Scholar]
- Ossification of the ligamentum flavum: Diet and genetics. J Clin Neurosci. 2007;14:703-5.
- [CrossRef] [Google Scholar]
- Phenotypic characterization of ligamentum flavum cells from patients with ossification of ligamentum flavum. Yonsei Med J. 2009;50:375-9.
- [CrossRef] [Google Scholar]
- Thoracic myelopathy caused by ossification of the ligamentum flavum. Clinicopathologic study and surgical treatment. Spine (Phila Pa 1976). 1991;16:280-7.
- [CrossRef] [Google Scholar]
- Uber interakuelle wirbelverkalkung. Fortschr Geb Rongenstr Nuklearmed Erganzungsband. 1920;40:292-8.
- [Google Scholar]
- Prevalence, distribution, and morphology of ossification of the ligamentum flavum: A population study of one thousand seven hundred thirty-six magnetic resonance imaging scans. Spine (Phila Pa 1976). 2010;35:51-6.
- [CrossRef] [Google Scholar]
- Dural ossification in ossification of the ligamentum flavum: A preliminary report. Spine (Phila Pa 1976). 2009;34:2654-61.
- [CrossRef] [Google Scholar]
- Symptomatic ossification of the ligamentum flavum: A clinical series from the French Antilles. Spine. 2005;30:E400-5.
- [CrossRef] [Google Scholar]
- The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87-100.
- [CrossRef] [Google Scholar]
- Spinal compression due to ossified yellow ligament: A short series of 5 patients and literature review. Surg Neurol. 2006;65:377-84. discussion 384
- [CrossRef] [Google Scholar]
- Thoracic myelopathy secondary to ossified ligamentum flavum. Acta Neurochir (Wien). 2001;143:775-82.
- [CrossRef] [Google Scholar]
- Frequency and size of ossifications in the caudal attachments of the ligamentum flavum of the thoracic spine. Role of rotatory strains in their development. An anatomic study of 121 spines. Surg Radiol Anat. 1992;14:119-24.
- [CrossRef] [Google Scholar]
- Thoracic myelopathy caused by ossification of the ligamentum flavum: Clinical features and surgical results in the Japanese population. J Neurosurg Spine. 2006;5:514-9.
- [CrossRef] [Google Scholar]
- Choice of operative method for ossification of ligamentum flavum based on CT findings [in Japanese] Rinsho Seikei Geka. 1996;31:541-5.
- [Google Scholar]
- MRI of ossification of ligamentum flavum. J Comput Assist Tomogr. 1992;16:73-6.
- [CrossRef] [Google Scholar]
- Variables affecting postsurgical prognosis of thoracic myelopathy caused by ossification of the ligamentum flavum. Spine J. 2013;13:1095-107.
- [CrossRef] [Google Scholar]
- MR T2 image classification in cervical compression myelopathy: Predictor of surgical outcomes. Spine (Phila Pa 1976). 2007;32:1675-8.
- [CrossRef] [Google Scholar]
- Cervical spondylotic myelopathy. Clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine (Phila Pa 1976). 1996;21:827-33.
- [CrossRef] [Google Scholar]
- Surgical approach to ossification of the thoracic yellow ligament. Surg Neurol. 1999;51:368-72.
- [CrossRef] [Google Scholar]
- Image-guided resection for thoracic ossification of the ligamentum flavum. J Neurosurg. 2003;99:60-3.
- [CrossRef] [Google Scholar]
- En bloc resection of lamina and ossified ligamentum flavum in the treatment of thoracic ossification of the ligamentum flavum. Neurosurgery. 2010;66:1181-6.
- [CrossRef] [Google Scholar]
- Surgical treatment of 40 patients with thoracic ossification of the ligamentum flavum. J Neurosurg Spine. 2006;4:191-7.
- [CrossRef] [Google Scholar]
- Thoracic myelopathy secondary to ligamentum flavum ossification. Ann Acad Med Singap. 2004;33:340-6.
- [CrossRef] [Google Scholar]
- Mechanism of destructive pathologic changes in the spinal cord under chronic mechanical compression. Spine (Phila Pa 1976). 2002;27:21-6.
- [CrossRef] [Google Scholar]







