Translate this page into:
The “Skull Flap” a new conceived device for decompressive craniectomy experimental study on dogs to evaluate the safety and efficacy in reducing intracranial pressure and subsequent impact on brain perfusion
Address for correspondence: Dr. Chibbaro Salvatore, Department of Neurosurgery, Strasbourg University Hospital, 1, Av Moliere, 67100, Strasbourg, France. E-mail: schibbaro@hotmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Decompressive craniectomy (DC) is a procedure performed increasingly often in current neurosurgical practice. Significant perioperative morbidity may be associated to this procedure because of the large skull defect; also, later closure of the skull defect (cranioplasty) may be associated to post-operative morbidity as much as any other reconstructive operation. The authors present a newly conceived/developed device: The “Skull Flap” (SF). This system, placed at the time of the craniectomy, offers the possibility to provide cranial reconstruction sparing patients a second operation. In other words, DC and cranioplasty essentially take place at the same time and in addition, patients retain their own bone flap. The current study conducted on animal models, represents the logical continuation of a prior recent study, realized on cadaver specimens, to assess the efficacy and safety of this recently developed device.
Materials and Methods:
This is an experimental pilot study on dogs to assess both safety and efficacy of the SF device. Two groups of experimental raised intracranial pressure animal models underwent DC; in the first group of dogs, the bone flap was left in raised position above the skull defect using the SF device; on the second group the flap was discarded. All dogs underwent transcranial Doppler (TCD) to assess brain perfusion. Head computed tomography (CT) scan to determine flap position was also obtained in the group in which the SF device was placed.
Results:
SF has proved to be a strong fixation device that allows satisfactory brain decompression by keeping the bone flap elevated from the swollen brain; later on, the SF allows cranial reconstruction in a simple way without requiring a second staged operation. In addition, it is relevant to note that brain perfusion was measured and found to be better in the group receiving the SF (while the flap being in a raised as well as in its natural position) comparing to the other group.
Conclusion:
The SF device has proved to be very easy to place, well-adaptable to a different type of flaps and ultimately very effective in maintaining satisfactory brain decompression and later on, making easy bone flap repositioning after brain swelling has subsided.
Keywords
Brain perfusion
cranioplasty
decompressive craniectomy
new device and technique
skull flap
trans-cranial Doppler
Introduction
Currently, decompressive craniectomy (DC) is carried out in cases of medically unresponsive raised intracranial pressure (ICP) as often seen in severe head injury, subarachnoid and intra-cerebral hemorrhage, malignant ischemic stroke, extensive cerebral venous sinus thrombosis and many other conditions responsible for life-threatening brain swelling (i.e., large tumors, etc.).[123456789101112] During the acute phase of brain swelling, a wide DC provides some degree of brain “protection" from likely irreversible damage.[13] Although, it may not reverse established neurological deficits. Usually, the bone flap is either stored in a bony bank under aseptic conditions or placed in the subcutaneous abdominal fat layer or is discarded; whenever the original bone flap is no longer available, then this may be reconstructed using various bio-materials (acrylic resin, porous hydroxyapatite, titanium plate, etc.) and replaced through cranioplasty. In either case, a second procedure becomes necessary. While DC is carried out in an emergent setting, a long and variable period of time goes by before reconstruction is planned, which is largely different going from 1 month to 12 months.[1415]
Recently, several studies have been published in literature about the influence of cranioplasty on clinical outcome.[1617181920212223] In 1977 Yamaura[16] reported that 30% of patients with a depressed skin flap following cranial decompression improved after cranioplasty. Globally, many authors have concluded, from their studies that cranial reconstruction is useful for not only cerebral protection, but also for the final patient functional outcome.[23] Others studies have shown that early cranioplasty would limit complications as hydrocephaly and epilepsy and improve neurological outcome allowing faster recovery.[11415212223] Up-to-date the main indication for cranioplasty still remains the direct brain protection from external injuries, the acceleration of patient's rehabilitation, to avoid the new onset of psychological problems due to poor cosmesis. Finally, it should bear in mind, the potential risks and elevated costs of a second and unavoidable procedure as cranioplasty.[2425262728]
The authors present a newly conceived device that offers at once the simultaneous benefits of a DC and reconstructive surgery. The safety and efficacy of such a device is now assessed on dogs, the present study representing the logical consequence of a previous feasibility study recently realized on cadaver specimen.
Materials and Methods
Description of system
The SF device is a permanent implant; it is manufactured according to International Organization for Standardization 5832-3 and it is made of Titanium ASTM F 136, grade 3.7165, tensile min 860, max 9999 Mpa that maintains optimal biocompatibility and is fully compatible with computed tomography (CT) and magnetic resonance imaging.
The SF consists of a hinge system that connects one edge of the removed bone flap to the skull. The opposite edge of the bone flap is connected to the skull by a plate/sliding track that carries a locking-unlocking system (photos 1); this plate is also connected to a titanium wire tunneled and externalized in the scalp at a site distant from the surgical and covered by a silicon tube. Within 2-4 weeks, when the brain swelling has subsided, the wire will serve as traction for the repositioning of the flap in its anatomical position. To unlock the system a peculiar maneuver is needed and specifically pulling the wire first downward and subsequently upward while it is right on the major flap axis this is to avoid any inadvertent/accidental release. The plate and the hinge also would allow bony fusion of the flap edges stabilizing and keeping the flap in place [Figure 1].
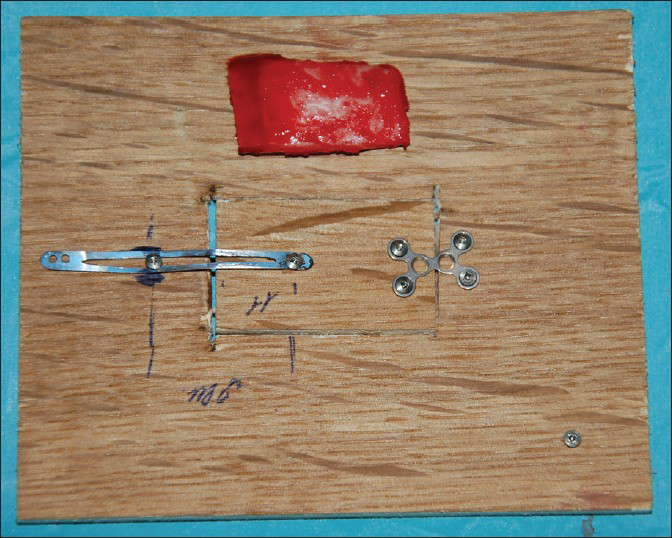
- Skull flap system model prototype developed in scale for dogs in the closed position with also the corresponding bone flap realized
Methods
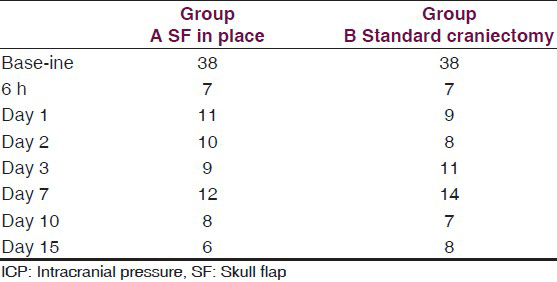
This is an experimental pilot study conducted on four female beagles of 13 months of age, weighing between 8.8 Kg and 11.2 Kg to assess the safety and efficacy of the SF device. The four dogs included in this study were used to create an experimental model of raised ICP; a small balloon was placed in the subdural space of each dog and inflated until a pressure of about 40 mmHg was reached. This was recorded with a Codman ICP monitoring system. Subsequently, the dogs were divided in two Groups (A and B) and underwent DC. Then the dogs in Group A underwent repositioning of the flap over the craniectomy defect using the SF device [Figure 2], whereas the dogs in Group B had the flap discarded [Figures 3 and 4]. ICP was monitored in all dogs postoperatively for the first 6 h after surgery, and then checked twice a day for the next 3 days and afterwards at day 7, 10 and 15 [Table 1]; at day 15 the balloon was deflated and removed. During these 15 days, all dogs underwent cerebral perfusion evaluation by TCD to determine if there was any difference between the two groups as well as to determine any difference between the ipsilateral/affected and contralateral/non affected side. All dogs underwent head CT at day 15 post-operatively; in Group A imaging were also completed before and after repositioning the flap in its anatomical position [Figures 5 and 6]. The bone flap was kept at a mean height of 25 mm from the outer skull edge, which was empirically chosen by the authors thinking that when performing a large craniectomy (i.e., 12 × 15) as they routinely do in real patient it could assure an adequate decompression gaining in the meantime a sufficient expansion volume thanking to the large surface.
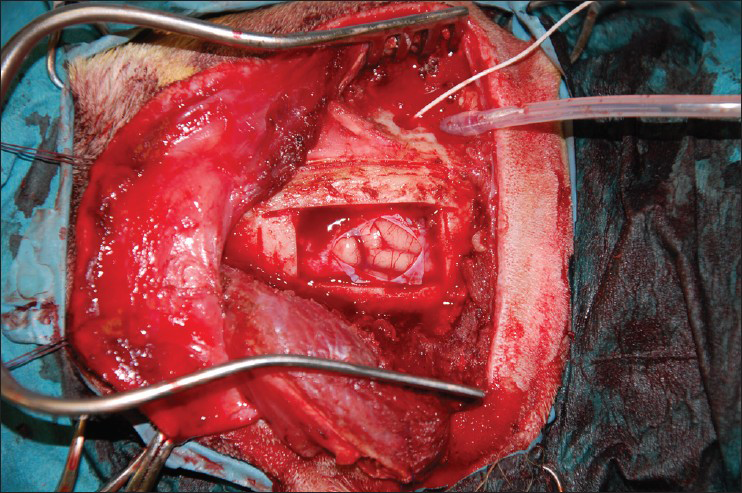
- Frontoparietal decompressive craniectomy showing the balloon and intracranial pressure monitor in situ
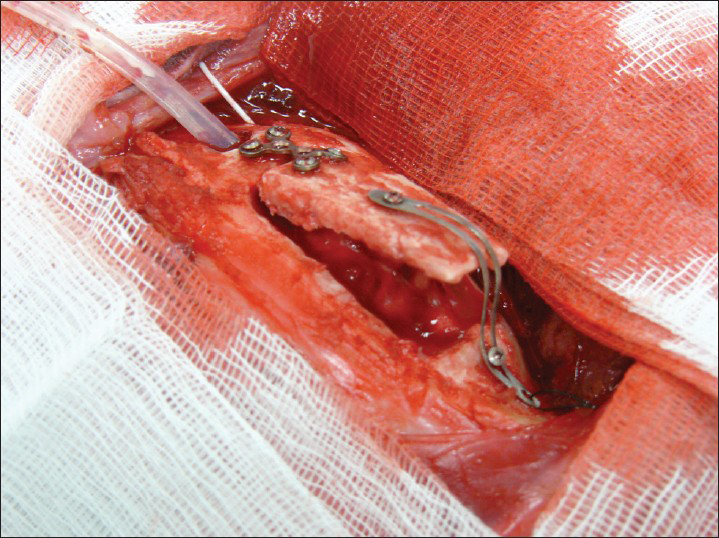
- Skull flap system in place in an open position
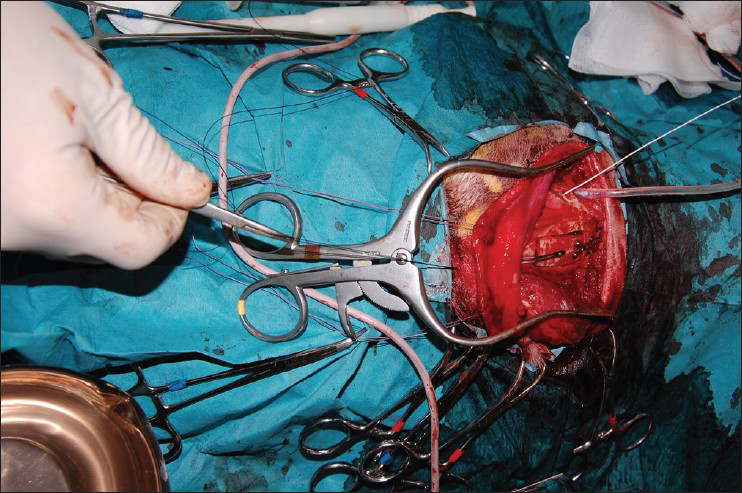
- Perioperative testing of skull flap device in repositioning the flap by pulling the wire
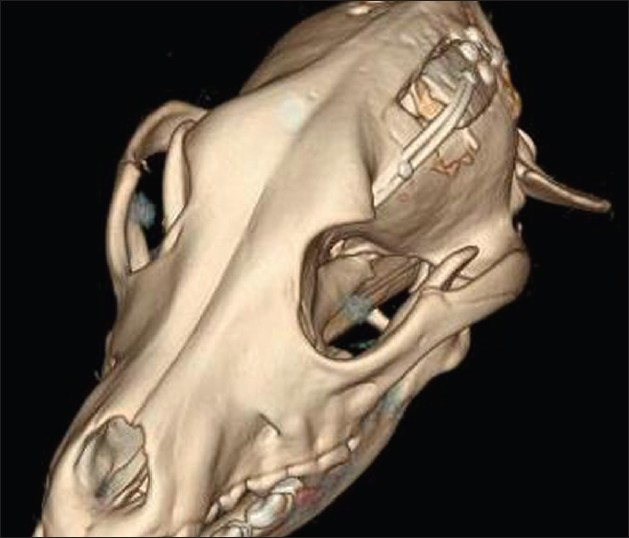
- 3D Post-operative computed tomography scan at day 15 showing skull flap system in the open position
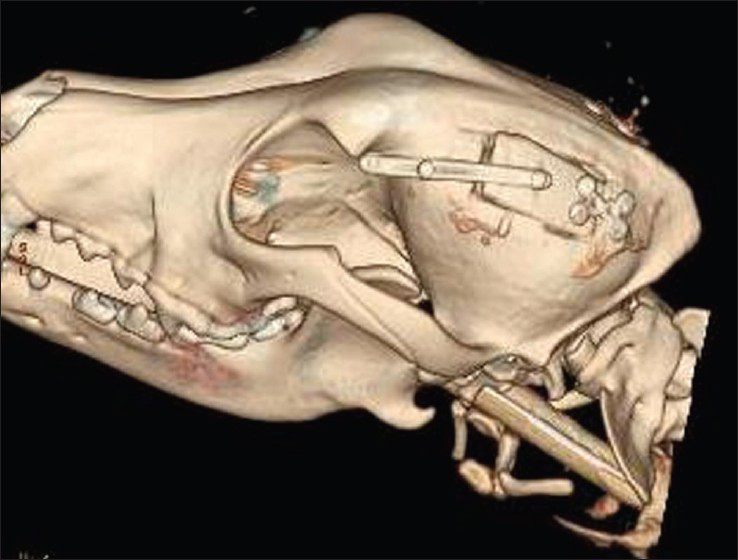
- Post-operative computed tomography scan at day 15 showing skull flap system in the closed position after pulling the wire
The present study has been performed in accordance with national guidelines for the care and use of animals and approved by the Paris National Veterinary School Hospital ethical committee (authorization number 94-046-2-09/2012).
Results
The dogs tolerated well the placement of the balloons and in Group A, placement of the SF device. The SF prototype evaluated in this study has shown to be very easy to use, adaptable and easy to place; the results, once again, clearly demonstrate that the SF is a solid fixation device that yet retains optimal plastic deformability and allows satisfactory and consistent brain decompression. In addition, the fact that an edge of the device remains directly connected to the skull allows the repositioning of the flap back in its natural place. The lock-unlock system has proved reliable and consistent flap descent once released. CT scans have shown more than acceptable repositioning of the flap. Needless to say, we were able to re-approximate perfectly the scalp over the raised flap when the flap was kept at a mean height of 25 mm (24-26 mm). Following DC, ICP was equally and permanently reduced in both groups of dogs from a mean of 38 mmHg (range 36-43) to a mean of 7 mmHg (range 6-8 see also table 1). TCD parameters were as follow: Group A: Various craniectomy side with flap left in raised position with SF: (Value as a mean) heart rate (HR): 110/min; middle cerebral artery (MCA) systolic velocity (Sv): 147 cm/s diastolic velocity (Dv): 117 cm/s IP: 0.22 ipsilateral/affected hemisphere; contralateral/non-affected hemisphere Sv: 145 cm/s Dv: 107 cm/s IP: 0.30. Group B: Various craniectomy side with flaps discarded: HR: 96/min Sv: 60 cm/s Dv: 35 cm/s IP: 0.4 ipsilateral/affected hemisphere; contralateral/non affected: Sv: 118 cm/s Dv: 52 cm/s IP: 0.77 [Graph 1].
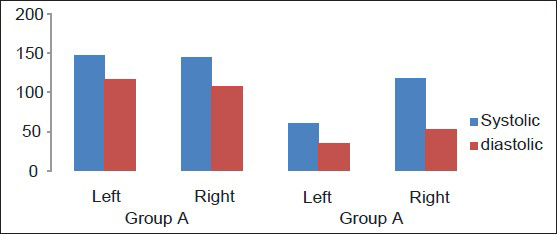
- Transcranial Doppler velocities comparison in dogs having left craniectomy
Discussion
DC is a procedure performed increasingly often as its indications have been extended to a number of conditions responsible for raised ICP intractable with medical management.[123456789101112] The main idea behind the SF system is that of having a device that allows DC while keeping the bone flap in place (desirably raised between 12 mm and 15 mm from the brain surface in a real patient) for later early replacement without the necessity of a second operation.
In this study, two objectives are reached and they can be so summarized as follow:
-
Satisfactory brain decompression reducing safely and effectively ICP
-
Repositioning of the flap in its anatomical position (usually after 2-4 weeks) without completing a second operation.
In addition, it should be mentioned that early skull flap (SF) replacement has also shown to favor a return of local brain perfusion as demonstrated by the increased internal carotid artery and MCA systolic and diastolic velocities associated with a reduction of the mean arterial blood pressure on Group A when compared with Group B. Additional benefits provided by the placement of the SF device include avoidance of all the problems related to the presence of a large skull defect such as direct brain injury, delayed patient's rehabilitation, patient's psychological problems due to poor cosmesis and above all, a second operation for cranial reconstruction.[232425262728] To our knowledge, there are no similar devices currently available in the international market. Some of the surgeons who has examined this device proposed the use of inverted “L" shaped mini-plates to keep the bone elevated; we disagree with this view as this technique would require re-intervention for their removal and repositioning of the bone flap; thus defying the purpose of the SF device; others have proposed to simply leave the bone flap floating on top the brain cortex as a simpler alternative;[29303132] in our opinion, leaving a “free floating" bone flap not only does not guarantee effective cerebral decompression, but it could also cause the formation of adhesions between the flap and the underlying tissues (brain and dura mater) preventing safe and proper later repositioning. Our analysis of the SF device reveals, it may have three relative limitations:
-
In some situation, it may be difficult to re-approximate the scalp over the elevated bone flap (although, we were able to close the skin on dogs in all craniectomies when the flap was kept raised with a mean of 25 mm)
-
Brain decompression may result less than optimal at the site where the bone flap is attached to the skull with the hinge; this problem prompted a modification of the SF device, which is still currently in progress. This is a modification that will permit equal bone flap elevation around the craniotomy edges thus allowing symmetrical and more effective decompression
-
The titanium wire exteriorized at the skin surface is covered by a silicon tube as for an ICP monitor thus, carrying possibly the same infection risk being practically very low.
Conclusion
The SF device has shown to be very easy, adaptable, and practical device to place. It is a strong fixation device retaining, at the same time, optimal plastic deformability allowing reliable brain decompression; this is so because the bone flap remains in an acceptable elevated position permitting sufficient brain expansion during the acute initial stages of brain swelling; then, it can be easily replaced back in its anatomical position by simply pulling a wire, once edema has subsided and thus, completing cranial reconstruction. SF presents also some limitation currently under study and technical modification. Our findings prompted additional experimental studies on a larger scale animal model to assess the safety of the SF device prior the development a clinical study to determine its real efficacy in humans.
Source of Support: Nil.
Conflict of Interest: The first (Salvatore Chibbaro) and last author (Paolo Diemidio) declare to be the inventor/patent of the device being implemented in the study.
References
- Decompressive craniectomy and early cranioplasty for the management of severe head injury: A prospective multicenter study on 147 patients. World Neurosurg. 2011;75:558-62.
- [Google Scholar]
- Role of decompressive craniectomy in the management of severe head injury with refractory cerebral edema and intractable intracranial pressure. Our experience with 48 cases. Surg Neurol. 2007;68:632-8.
- [Google Scholar]
- Combined internal uncusectomy and decompressive craniectomy for the treatment of severe closed head injury: Experience with 80 cases. J Neurosurg. 2008;108:74-9.
- [Google Scholar]
- Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506-17.
- [Google Scholar]
- Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215-2.
- [Google Scholar]
- Surgical decompression for traumatic brain swelling: Indications and results. J Neurosurg. 1999;90:187-96.
- [Google Scholar]
- EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien). 1997;139:286-94.
- [Google Scholar]
- Epidemiology of severe head injury: Socioeconomic consequence of avoidable mortality and morbidity. In: Scriabine A, Teasdale GM, Tettenborn D, Young W, eds. Nimodipine: Pharmacological and Clinical Results in Cerebral Ischemia. Berlin: Springer; 1991. p. :225-33.
- [Google Scholar]
- Primary decompressive craniectomy in patients with aneurysmatic subarachnoid hemorrhage. Results of a pilot study in 11 cases. Neurocirugia (Astur). 2010;21:452-60.
- [Google Scholar]
- Decompressive surgery in cerebrovenous thrombosis: A multicenter registry and a systematic review of individual patient data. Stroke. 2011;42:2825-31.
- [Google Scholar]
- Should decompressive surgery be performed in malignant cerebral venous thrombosis?: A series of 12 patients. Stroke. 2010;41:727-31.
- [Google Scholar]
- Enhanced vulnerability to secondary ischemic insults after experimental traumatic brain injury. New Horiz. 1995;3:376-83.
- [Google Scholar]
- Improved brain protection at decompressive craniectomy: A new method using Palacos R-40 (methylmethacrylate) Acta Neurochir (Wien). 2005;147:279-81.
- [Google Scholar]
- Expanded polytetrafluoroethylene membrane for prevention of adhesions in patients undergoing external decompression and subsequent cranioplasty. Neurol Med Chir (Tokyo). 2003;43:320-3.
- [Google Scholar]
- Neurological deficits in the presence of the sinking skin flap following decompressive craniectomy. Neurol Med Chir (Tokyo). 1977;17:43-5.
- [Google Scholar]
- Hemodynamic and metabolic effects of decompressive hemicraniectomy in normal brain. An experimental PET-study in cats. Brain Res. 2003;982:31-7.
- [Google Scholar]
- CT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplasty. Clin Neurol Neurosurg. 2006;108:583-5.
- [Google Scholar]
- Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93:53-61.
- [Google Scholar]
- The influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. Neurosurg Focus. 2000;8:e9.
- [Google Scholar]
- The impact of early cranioplasty on cerebral blood flow and its correlation with neurological and cognitive outcome. Prospective multi-centre study on 24 patients. Rev Neurol (Paris). 2013;169:240-8.
- [Google Scholar]
- The impact of early cranioplasty on cerebral blood flow and metabolism and its correlation with neurological and cognitive outcome: Prospective multi-center study on 34 patients. Indian J Neurosurg. 2012;1:17-22.
- [Google Scholar]
- Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26:E7.
- [Google Scholar]
- Cranioplasty after decompressive craniectomy: An institutional audit and analysis of factors related to complications. Surg Neurol Int. 2011;2:123.
- [Google Scholar]
- Significance of hydrocephalus following severe brain injury during post-acute rehabilitation. Ideggyogy Sz. 2010;63:397-401.
- [Google Scholar]
- Long-term complications of decompressive craniectomy for head injury. J Neurotrauma. 2011;28:929-35.
- [Google Scholar]
- Cranioplasty after postinjury decompressive craniectomy: Is timing of the essence? J Trauma. 2010;69:270-4.
- [Google Scholar]
- Four-quadrant osteoplastic decompressive craniotomy: A novel technique for decompressive craniectomy avoiding revision cranioplasty after surgery. Neurol India. 2012;60:672-4.
- [Google Scholar]
- Decompressive craniectomy bone flap hinged on the temporalis muscle: A new inexpensive use for an old neurosurgical technique. Surg Neurol Int. 2011;2:150.
- [Google Scholar]
- Cranioplasty with bone flaps preserved under the scalp. Neurosurg Rev. 1996;19:153-6.
- [Google Scholar]






