Translate this page into:
The Effect of Statins in Epilepsy: A Systematic Review
Address for correspondence: Dr. Luis Rafael Moscote-Salazar, University of Cartagena, Cartagena de Indias, Colombia. Centro de Investigaciones Biomédicas, Faculty of Medicine, University of Cartagena, Cartagena de Indias, Colombia. E-mail: mineurocirujano@aol.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background and Objectives:
Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, used for the management of hypercholesterolemia and related atherosclerotic diseases. Several studies have indicated the neuroprotective effects of statins on several neuropathological conditions. However, the role of these medications in epilepsy is still unclear. The purpose is to evaluate and summarize the level of evidence on the efficacy of statins in neuronal hyperexcitability and the neuroinflammatory processes of epilepsy.
Methods:
A systematic review was performed. Eligibility Criteria: This review involved studies conducted in humans and nonhuman experimental models, covering the use of an inhibitor of HMG-CoA reductase, alone or accompanied by another medication, in epilepsy. Information Sources: A systematic literature search was performed in PubMed, Embase, Ebsco Host, Scopus, Science Direct, Medline, and LILACS. Risk of Bias: It was evaluated with the Newcastle–Ottawa Scale and the experimental studies were evaluated using the GRADE tool.
Results:
Twenty articles of the 183 evaluated were included. Sixteen studies were conducted in animal models and four studies in humans. Most studies in mice reported a reduction in epileptiform activity and reduction in systemic inflammation with the treatment of statins, potentially influencing epilepsy control. Few studies in humans were performed in the geriatric population with variable results (neuroinflammation, seizure prevention, cell death, prevention of kindling, increase in convulsive threshold, increase in latency, decrease in frequency of crisis, and reduction in mortality) related to reduction in the rate of hospitalizations, mortality, and prevention of epilepsy. Studies in mice found a decrease in interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha and an increase in IL-10 and endothelial nitric oxide synthase.
Conclusions:
The possible antiepileptic mechanism of statins may be related to the reduction in neuroinflammation mediated by a decrease in pro-inflammatory cytokines and action in the nitrergic system. Further studies evaluating the impact of statins on seizure control are necessary.
Keywords
Brain
epilepsy
seizure
statins
INTRODUCTION
Epilepsy is a neurological disease generated by an abnormal brain electrical activity in certain regions of the brain that predisposes to recurrent unprovoked attacks. Worldwide, a prevalence of 1%–2% is estimated, affecting between 50 and 65 million people, with 50,000–100,000 new cases per year. In addition, it is estimated that 30%–40% are refractory to seizure treatment.[123] Epilepsy is considered a public health problem worldwide and is one of the most frequent neurological disorders.[3]
Statins are inhibitors of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase, used for the management of hypercholesterolemia and related atherosclerotic diseases, such as coronary artery disease.[145] The enzyme HMG-CoA reductase catalyzes the conversion of HMG-CoA to L-mevalonate; statins prevent the biological activities of L-mevalonate due to the aforementioned inhibitory effect.[5] Statins perform pleiotropic actions on the endothelium, the inflammatory response, or the production of free radicals. The inhibition of obtaining endogenous cholesterol induces a positive regulation of low-density lipoprotein (LDL) receptors on the cell surface. Obtaining a higher absorption of LDL from the blood and therefore a decrease in its concentration. In addition, high-density lipoprotein levels increase and triglyceride levels decrease.[6]
Several studies have indicated the neuroprotective effects of statins on several neuropathological conditions. The anticonvulsive activity has been explained by suppressing reactive astrogliosis with neuroinflammation in the crises; by stimulating GABAergic activity and inhibiting glutamatergic; by modulating the glycogen synthase kinase-3β pathway; and by decreasing the infiltration of monocytes in the neuronal death of the hippocampus and pro-inflammatory gene expression.[14]
The lipophilic statins (atorvastatin [ATV], lovastatin, fluvastatin, pitavastatin, and simvastatin) can passively pass through the blood–brain barrier (BBB); in addition, the hydrophilic statins can also enter the neuroparenchyma.[67] All statins are substrates for organic anion transporter polypeptides (OATPs), of which OATP1A2 and OATP1C1 are expressed in the brain. Despite this, the selectivity of hydrophilic statins for these subtypes has not been explored to determine their mechanism of entry to the central nervous system (CNS). In addition, the existence of monocarboxylic acid transporters in the BBB can constitute an alternate route of entry to the CNS although there are no specific studies for the CNS either. Independently of the specific transporters, it is feasible that statins are deposited at different speeds and concentrations within the CNS according to their different lipid solubility alone.[7]
METHODS
Objectives
The objective of this review is to answer the following question: What is the level of evidence on the efficacy of statins to decrease neuronal hyperexcitability and neuroinflammatory processes of epilepsy?
To develop the review, the steps of the patients, intervention, comparison, outcomes, and study design strategy were followed.
Inclusion criteria
Types of participants
This review involved studies conducted in humans and nonhuman experimental models.
Type of intervention
It covered the use of inhibitors of HMG-CoA reductase, alone or accompanied by another medication.
Types of studies
The qualitative component of the review included studies that described the molecular mechanisms of statins in the CNS, the pharmacokinetic aspects, and the changes derived from the use of these drugs in neurotransmitters and cerebral cholesterol. The quantitative component included randomized and nonrandomized controlled trials, quasi-experimental designs, prospective or retrospective cohort studies, and nested case–control studies.
Types of results
The benefit of the therapy was defined as the decrease in neuroinflammation derived from seizures, decrease in neuronal death after the seizure, prevention of seizures, decrease in mortality after a seizure episode, increase in seizure threshold, reduction in the frequency, and increase in the latency of the crises.
Search and selection of studies strategy
This systematic review followed the recommendations of the Cochrane Collaboration (PRISMA). A bibliographic search was carried out in the databases: PubMed, Embase, Ebsco Host, Scopus, ScienceDirect, Medline, and LILACS, considering all the publications made up to February 2, 2018. The search was carried out in five steps: First, the keywords using the Medical Subject Headings and DeCs (Health Descriptors), then proceeded to use the descriptors of the subject (hydroxymethyl glutaryl-CoA reductase inhibitors, statins, epilepsy, epileptogenesis) in the databases mentioned. Subsequently, the duplicate records were eliminated (Step 2) and an analysis of the titles and abstracts thrown by the search was continued (Step 3), then the full-text review of the selected articles was proceeded (Step 4), and finally, the references were reviewed of the included articles to identify those studies that also met the eligibility criteria (Step 5). Letters to the editor and studies with a language other than English or Spanish were excluded.
Method of revision
The search strategy and selected studies were evaluated by two independent reviewers (LM and ZC). The discrepancies were discussed with a third reviewer (RM).
Data collection
The following data were extracted from the studies that met the eligibility criteria: authors, year of publication, number of participants, type of study, study objective, statin used, statin dose, epilepsy inducer used, route of administration of the statin, duration of treatment, and results of the study. These data were compiled in Microsoft Excel and were divided into two: collection of human studies and collection of non-human studies.
Assessment of quality and risk of study sessions
The assessment of the risk of bias in human studies was performed using the Newcastle–Ottawa Scale [Figures 1 and 2], while experimental studies in animal models were evaluated using the GRADE tool [Figure 3].

- PRISMA flow diagram of our search mechanism
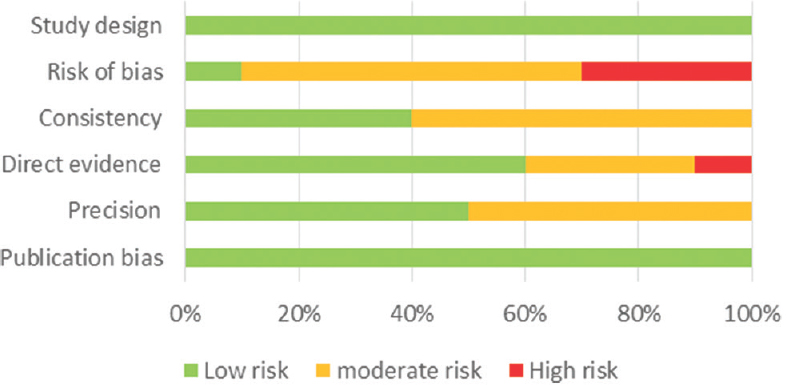
- Risk of bias for experimental studies in animal models using GRADE
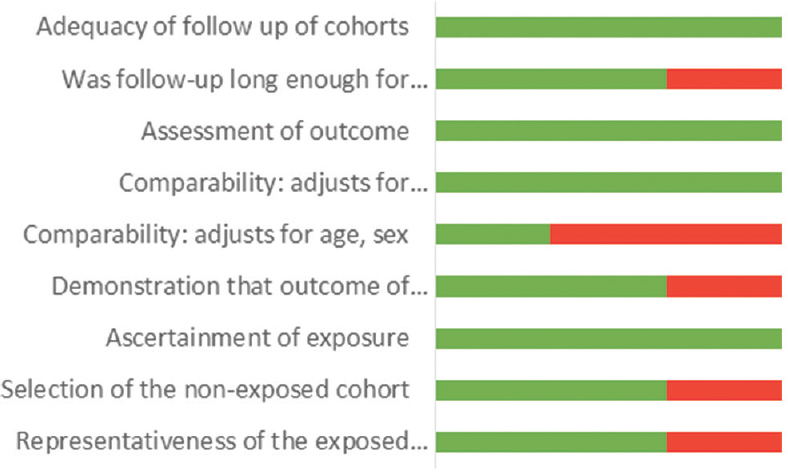
- Risk of bias using Newcastle–Ottawa Scale for cohort studies
Data synthesis and additional analysis
An analysis of the studies was carried out in nonhuman experimental models that only involved the use of statins, excluding from this analysis those studies that applied a drug or substantial addition to the statin and the studies that did not specify the duration of the treatment. With the Epi-info 7.2 programs, (CDC, Atlanta, Georgia, USA) the median duration of treatment, absolute and relative frequency of the variables were determined: statin used, statin dose, epilepsy inducer used, and route of administration.
RESULTS
Selection of studies
The selection process of the studies was based on the PRISMA foundations as shown in Figure 4. Twenty articles of the 183 included in the bibliographic search were considered.
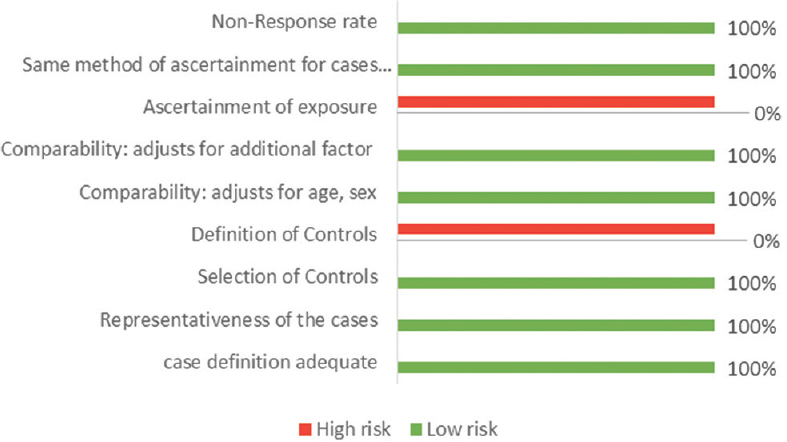
- Risk of bias using Newcastle–Ottawa Scale for case–control studies
Characteristics of the studies
Studies in animal models
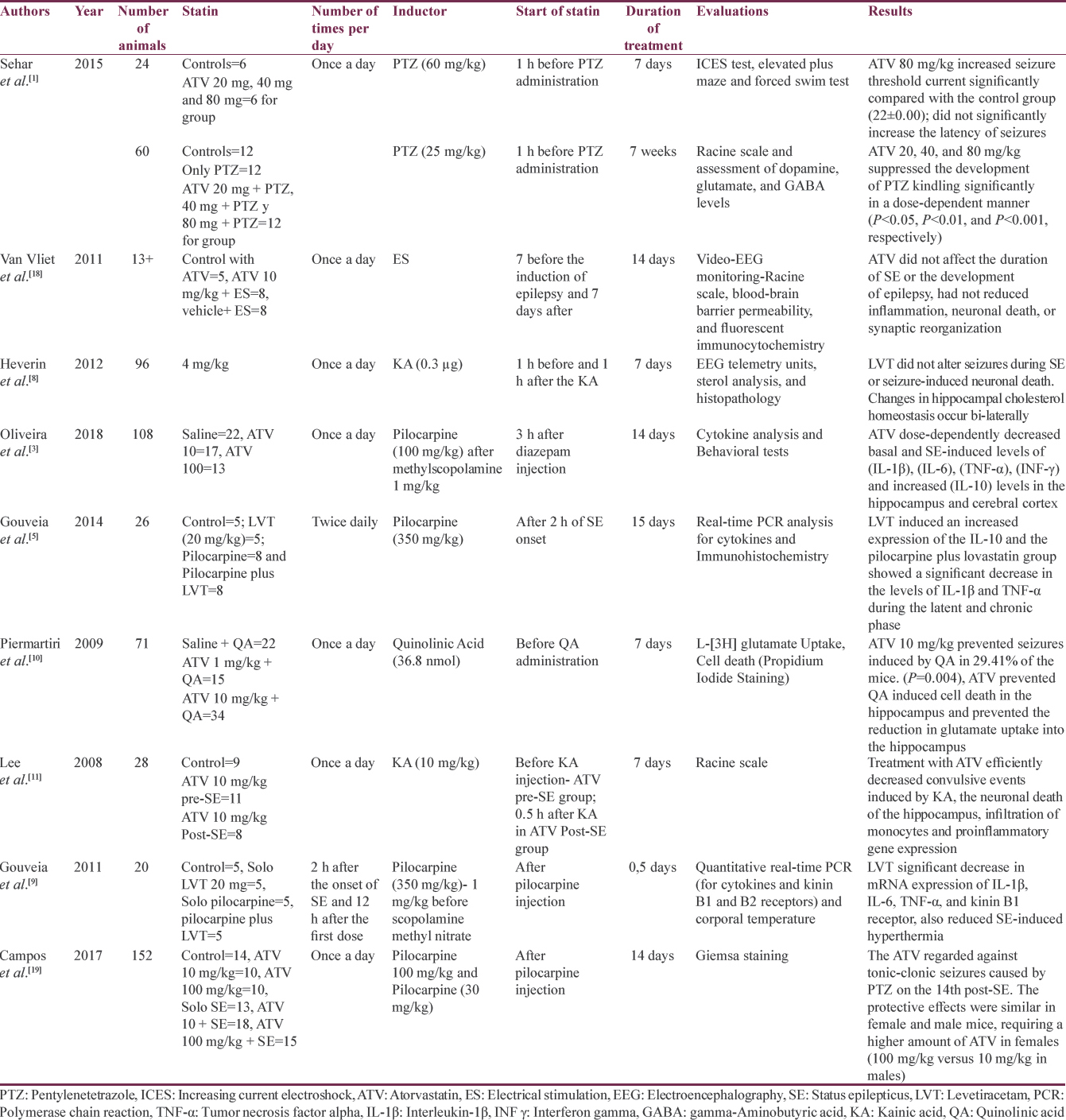
We identified 16 studies in animal models (mice), of this total only 10 studies (n = 626 mice) met the criteria for descriptive analysis [Table 1]. We excluded three studies that used an additional substance to the statin; one study that did not clearly specify the duration of the treatment and two studies that did not clarify the number of participants. The median duration of treatment with statins was 14 days (IQR = 7–15), the inducers of epilepsy used were pilocarpine, kainic acid, quinolinic acid, pentylenetetrazole (PTZ), and electroshock test, as shown in Figure 5. Sehar et al.'s study was performed in two phases: acute and chronic, for statistical analysis were taken as two studies. On the other hand, as can be seen Figure 6, the most used route of administration was oral. In addition, the most commonly used statin dose was 10 mg of ATV [Figure 7]. In Table 2, it is observed that most of the studies reported a positive change of statin treatment.
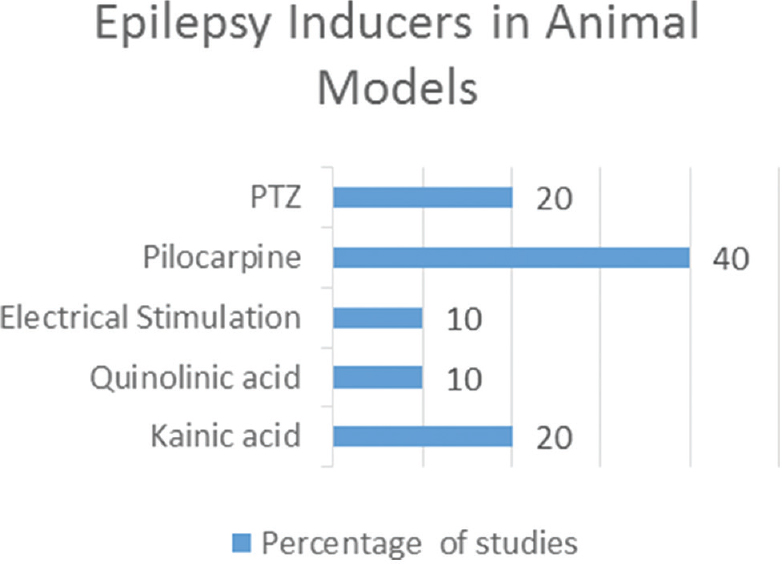
- Epilepsy inducers
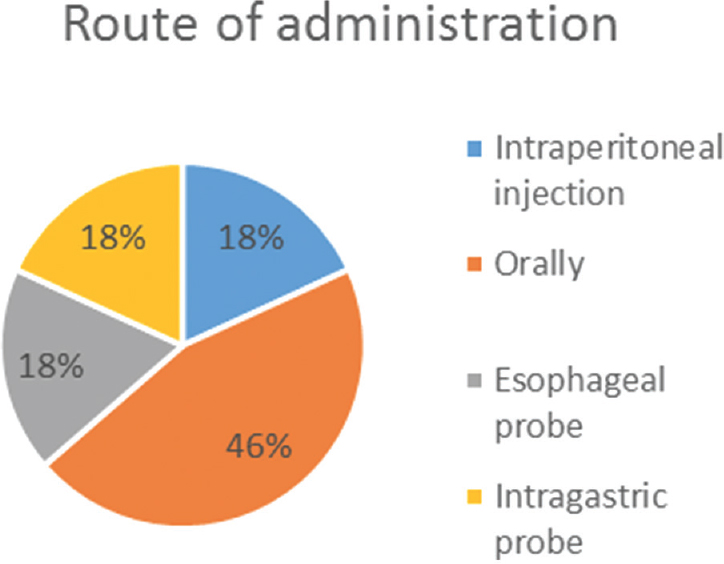
- Statin route of administration
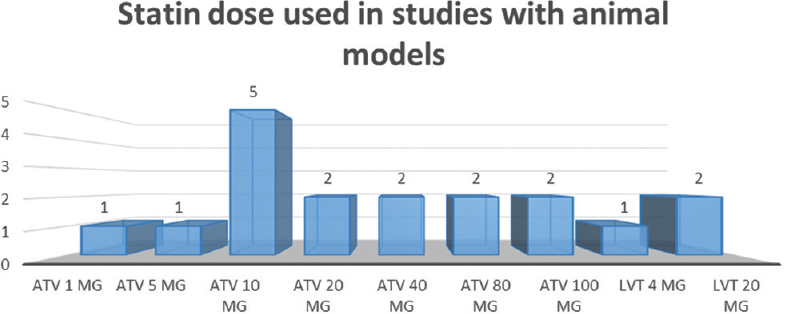
- Number of studies and dose of statin used in the experimental studies
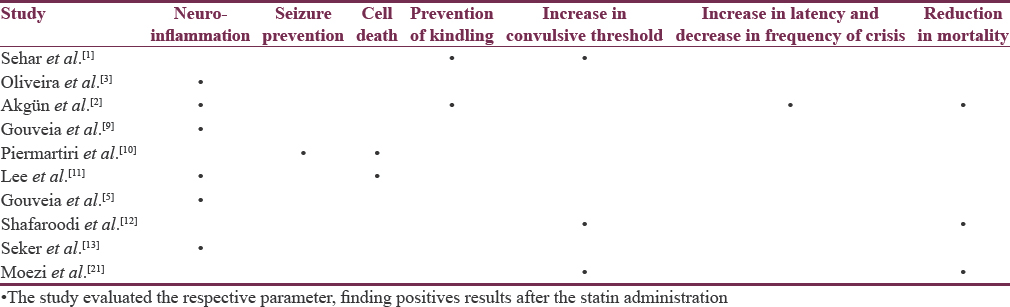
Human studies
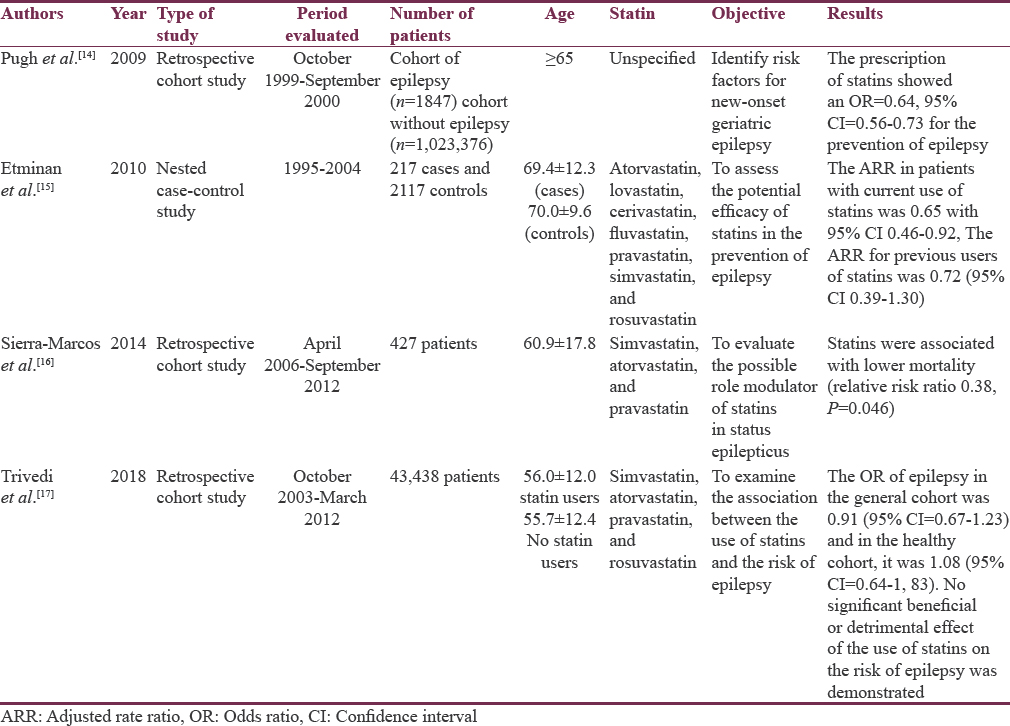
We identified four studies in humans (n = 1,071,422): 3 (75%) of retrospective cohort and 1 (25%) of nested cases and controls. The results are shown in Table 3.
Evaluation of the risk of bias
The results of the evaluation of the bias are shown in Figures 2–4.
Statins and brain cholesterol
One study quantified sterol levels in the hippocampus of an animal model with unilateral hippocampal lesion induced by kainic acid. The lovastatin treatment was performed 3 days before and 3 days after the induction of status epilepticus (SE). There were no significant changes in the levels of sterols (lanosterol and desmosterol) at 24 h after the SE, but it did detect a bilateral reduction in the hippocampus of the metabolite 24-OHC and cholesterol levels at 48 h and 14 days (P < 0.001 and P < 0.01, respectively). These periods in which changes were identified corresponded to the preepileptogenic and to the appearance of daily seizures. On the other hand, lovastatin was not associated with alteration of seizures during status.[8]
Statins and neuroinflammation
We identified three studies related to the role of statins in neuroinflammation mediated by cytokines. Two studies used lovastatin and one study used ATV. All three studies administered postinduction statin with pilocarpine. The study by Oliveira et al. found a reduction of the pro-inflammatory cytokines: interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha (TNF-α), and interferon-γ in cortex and hippocampus, 14 days after induction (P < 0.05) together with an increase in the levels of the anti-inflammatory cytokine IL-10. These results were better with the dose of ATV 100 mg/kg compared to the dose of 10 mg/kg. In addition, the control group of ATV without induction with pilocarpine also showed a reduction in pro-inflammatory cytokines. In this same study, it was found that mice needed higher doses of the statin to achieve an increase in IL-10, unlike rats, which in turn had higher levels of pro-inflammatory cytokines.[3] Gouveia et al.found similar results in both 2011 and 2014.[59] The two studies used ATV with a difference in the duration of treatment. However, both studies found a reduction in IL-1β and TNF-α.[59]
Statins and nitric oxide
Four studies evaluated the effect of statins on nitric oxide. Three studies used PTZ as an inducer and one used penicillin G ATV.[13161720] Akgün Dar et al, administered ATV 30 min before induction found that pretreatment with this statin reduced the inducible nitric oxide synthetase and matrix metalloproteinase 2 that have been related to a proconvulsant activity (P < 0.001) and also found a reduction in frequency and an increase in latency of seizures (P < 0.001).[2] These findings agree with the results of the study by Shafaroodi et al., who used PTZ and electrical stimulation as inductors and administered ATV of 10 and 20 mg/kg; this treatment increased the threshold for tonic seizures caused by PTZ and decreased the appearance of tonic seizures and death in the induction model with electric current.[12] Similarly, the study by Moezi et al. found an increase in the seizure threshold for both intravenous and intraperitoneal PTZ models and a decrease in the appearance of tonic seizures and death in the chronically treated group with ATV and induction with intraperitoneal PTZ.[21] In the study by Seker et al., ATV, simvastatin, and rosuvastatin were used at a dose of 20 mg/kg; in this case, the group with rosuvastatin presented the best antiepileptic effect with a decrease in the expression of p53, Bax, and caspase 3 that are associated with cellular apoptosis, together with an increase in endothelial nitric oxide synthetase.[13] Three studies (Seker et al., Shafaroodi et al., and Moezi et al.) showed that inhibitors of nitric oxide synthetase (L-NAME and aminoguanidine) decreased the anticonvulsant effect of ATV.[121321]
Statins and specific syndromes associated with epilepsy
We identified two studies that evaluated the use of statins in genetic syndromes with epileptogenic characteristics. In 2013, Osterweil et al. found that the administration of 100 mg/kg of lovastatin to genetically modified mice (fragile X syndrome) was able to correct the excessive synthesis of proteins and prevent the outbreak of epileptiform activity in the hippocampus in vitro, in addition to protecting the mice in vivo.[122] On the other hand, in 2018, it was postulated that lovastatin is capable of modulating upregulated protein functions in animal models with Angelman syndrome by a mechanism other than the inhibition of protein synthesis.[23] However, more research is needed to clarify this relationship.
DISCUSSION
The neuroprotective effects of statins have been observed in various neurological diseases. However, its protective effect on epilepsy continues to be debated and most studies have been conducted in rats. An association has been found between the use of statins and the reduction of the risk of epilepsy in older adults. Sierra-Marcos et al. demonstrated an antiepileptic role of statins in older adults, for which they used the registry of 427 patients with an epileptic episode in a period of 6 years and took into account different predictive items of prognosis, among which if they had used or they use statins. The use of statins was statistically significant, which correlated with a decrease in morbidity and mortality in these patients.[16]
Etminan et al. found that statins reduce the risk of hospitalization in patients with epilepsy focused on senile patients and stipulated that the result is directly related to the dose and the possible anti-inflammatory properties that statins confer.[15] The few studies conducted in humans focused on observing the properties of statins in terms of epilepsy have had an effect in elderly people who have been shown to have a certain risk of developing epilepsy associated with cerebrovascular diseases and dementia. Pugh et al., in their study on the risk factors of epilepsy of onset in old age, observed that in patients with prescription of statins, the development of epilepsies was lower.[14]
There is research in humans that suggests the neuroprotective benefit of statins that is generalized to the entire population. Trivedi et al. could not determine the benefit of statins in their study where they included healthy individuals or those with few comorbidities and who used statins or not; however, it is emphasized that they were not associated with an increase in the risk of statins. Development of epilepsies which allows establishing that the use of statins is safe in patients who have epilepsy.[17] All studies reviewed in humans agree on the need for more research on the subject to be able to define the beneficial effect of statins and be able to explain it.
Summary of evidence
This systematic review gives an overview of the available literature on the role of statins and its possible effect on epilepsy. This study only provides evidence 3b.
Limitations
Our study has some limitations. Most studies focused largely on an experimental level. All articles included in this review are peer-reviewed. There is a possibility of publication bias. Finally, the inclusion of only articles in English and Spanish could affect the generalization of our findings.
CONCLUSIONS
The role as anticonvulsant agents of statins is not completely known. Still, there is missing evidence to know how this type of mechanism works modulating the immune system. The participation of statins as reducing agents of neuroinflammation requires more studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Atorvastatin prevents development of kindling by modulating hippocampal levels of dopamine, glutamate, and GABA in mice. Epilepsy Behav. 2015;42:48-53.
- [Google Scholar]
- Evaluation of antiepileptogenic effect of atorvastatin on development of pentylenetetrazole induced kindling in rats: The responsibility of inducible nitric oxide synthase and metalloproteinase 2. Turk Klin J Med Sci. 2013;33:995-1006.
- [Google Scholar]
- Effect of atorvastatin on behavioral alterations and neuroinflammation during epileptogenesis. Epilepsy Behav. 2018;78:109-17.
- [Google Scholar]
- Protective effects of some statins on epileptogenesis and depressive-like behavior in WAG/Rij rats, a genetic animal model of absence epilepsy. Epilepsia. 2014;55:1284-91.
- [Google Scholar]
- Lovastatin decreases the synthesis of inflammatory mediators during epileptogenesis in the hippocampus of rats submitted to pilocarpine-induced epilepsy. Epilepsy Behav. 2014;36:68-73.
- [Google Scholar]
- Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. 2014;15:20607-37.
- [Google Scholar]
- Bi-lateral changes to hippocampal cholesterol levels during epileptogenesis and in chronic epilepsy following focal-onset status epilepticus in mice. Brain Res. 2012;1480:81-90.
- [Google Scholar]
- Lovastatin decreases the synthesis of inflammatory mediators in the hippocampus and blocks the hyperthermia of rats submitted to long-lasting status epilepticus. Epilepsy Behav. 2011;20:1-5.
- [Google Scholar]
- Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-β(1-40) administration in mice: Evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol. 2010;226:274-84.
- [Google Scholar]
- Statin inhibits kainic acid-induced seizure and associated inflammation and hippocampal cell death. Neurosci Lett. 2008;440:260-4.
- [Google Scholar]
- The involvement of nitric oxide in the anti-seizure effect of acute atorvastatin treatment in mice. Neurol Res. 2012;34:847-53.
- [Google Scholar]
- HMG-coA reductase inhibitor rosuvastatin improves abnormal brain electrical activity via mechanisms involving eNOS. Neuroscience. 2015;284:349-59.
- [Google Scholar]
- New-onset epilepsy risk factors in older veterans. J Am Geriatr Soc. 2009;57:237-42.
- [Google Scholar]
- Statin use and risk of epilepsy: A nested case-control study. Neurology. 2010;75:1496-500.
- [Google Scholar]
- Statins are associated with decreased mortality risk after status epilepticus. Eur J Neurol. 2015;22:402-5.
- [Google Scholar]
- Association of statin therapy with risk of epilepsy in 2 propensity score-matched cohorts. Ann Pharmacother. 2018;52:546-53.
- [Google Scholar]
- Atorvastatin treatment during epileptogenesis in a rat model for temporal lobe epilepsy. Epilepsia. 2011;52:1319-30.
- [Google Scholar]
- Human lagochilascariasis – A rare helminthic disease. PLoS Negl Trop Dis. 2017;11:e0005510.
- [Google Scholar]
- Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31:1443-51.
- [Google Scholar]
- Chronic administration of atorvastatin induced anti-convulsant effects in mice: The role of nitric oxide. Epilepsy Behav. 2012;23:399-404.
- [Google Scholar]
- Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77:243-50.
- [Google Scholar]
- Lovastatin suppresses hyperexcitability and seizure in angelman syndrome model. Neurobiol Dis. 2018;110:12-9.
- [Google Scholar]






