Translate this page into:
The Challenges of Management of High-grade Gliomas in Nigeria
Address for correspondence: Dr. Chika Anele Ndubuisi, Memfys Hospital for Neurosurgery, P. O. Box: 2292, Enugu, Nigeria. E-mail: chikandu@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
High-grade gliomas (HGG) are among the most challenging brain tumors despite many research efforts worldwide.
Aim:
The aim of this study was to evaluate the local challenges that may influence outcome of HGG managed in a neurosurgical center in Nigeria.
Methodology:
Retrospective analysis of prospectively recorded data of patients managed for intracranial HGG at Memfys Hospital for Neurosurgery, Enugu, Nigeria, between the year 2006 and 2015. Only cases with conclusive histology following surgery were analyzed.
Results:
Glioma was 60 (23.8%) of 252 histology confirmed brain tumors. HGG represented 53.8% of gliomas with male:female ratio of 2.2:1.0 and peaked from fifth decade of life. Glioblastoma multiforme accounted for 69% of HGG. At 1-year postsurgery, 53% of HGGs were dead and 88% of these deaths were in the World Health Organization Grade IV group. Only 40% of cases could receive adjuvant treatment with only 15% mortality at 1 year in this subgroup that received adjuvant therapy. In addition, 19% of cases had surgery at Karnofsky score (Ks) of ≥70%. However, 94% of mortality at 1 year was related to surgery at Ks of ≤60%. Only four patients had a tumor volume of ≤50 cm3, and among these cases, three patients were independent at 1 year. Patients with tumor volume above 50 cm3 accounted for 94% of mortality.
Conclusion:
The peak age incidence for HGG seems to be lower than in Caucasians. Most cases present late with poor Ks and big tumor volume. The proportion with access to adjuvant treatment is still poor. Preoperative Karnofsky, extent of resection, duration of hospital, and Intensive Care Unit stay have impact on outcome.
Keywords
Challenges
Enugu
high-grade glioma
outcome
INTRODUCTION
Gliomas are the most common brain tumors in most parts of the world. In Nigeria, it is next to meningioma in frequency of occurrence and represents between 20% and 25% of brain tumors.[123] High-grade glioma (HGG) remains the most challenging brain tumors in spite of extensive research efforts and funds channeled into understanding the natural history and management of the disease. Treatment decisions remain controversial, and protocols vary with available resources.[456] Although extent of surgical resection and appropriate and adequate adjuvant chemo- and radiotherapy have been argued to offer the best chance of good management outcome,[78910] success of treatment is also impacted by factors such as delay in presentation, tumor size, the clinical status at time of presentation and availability of these adjuvant therapeutic options peculiar to low-resource centers. In Nigeria, few available studies have focused on gliomas in general and there is a need for local studies that will highlight the local challenges and other factors affecting the outcome of HGGs. The aim of this study is to analyze the outcome of HGGs managed in Enugu, Nigeria, and to highlight some local challenges that affect the outcome of HGGs in Nigeria.
Methodology
This study is a retrospective analysis of prospectively recorded data of patients managed for intracranial HGGs at Memfys Hospital for Neurosurgery (MHN), Enugu, Nigeria. MHN is a private postgraduate training neurosurgery center serving the southern part of Nigeria. The study period was from years 2006 to 2015. Following clinical review, the diagnosis and anatomical location of the lesion were confirmed in all patients with both noncontrast and postcontrast-enhanced brain magnetic resonance imaging (MRI) scan. Brain computed tomography (CT) scan was accepted for diagnosis in patients managed earlier in the study period (2005–2009) when the hospital did not have MRI machine. All the cases analyzed in this study had surgery. Demography, imaging finding, clinical history, surgery details, anatomy location, tumor size, the histology grade, and clinical outcome were analyzed. Tumor size estimation was measured as one-half of the value of the widest dimension in anterior-posterior, transverse, and height of the lesion in millimeters from MRI or CT scan. However, we excluded patients operated upon with histological diagnosis of low-grade glioma, other brain tumors and cases without a conclusive histology report from this study. The World Health Organization (WHO) classification was used for the tumor grading.[11]
Following neurosurgical intervention, all the patients were managed in the Intensive Care Unit (ICU) in the immediate postoperative period and later transferred to the ward for optimization. At discharge, the patients were transferred to secondary facilities for rehabilitation. Anticonvulsants and steroids were administered to all patients before surgery. Anticonvulsants were continued for minimum of 6 months before being tailed off while the steroids were generally continued until completion of radiotherapy and tailed off. Occasionally, steroids were continued for longer periods if patients still had persisting vasogenic edema following surgery or radiotherapy. All patients with HGG were referred to oncologist for adjuvant therapy using radiotherapy and temozolomide.
All patients had postoperative neuroimaging investigation before discharge from the hospital. The clinical follow-up protocol was at 4–6 weeks, 3 months, 6 months, and 1 year and subsequently yearly. Neuroimaging follow-up investigations were obtained when clinically indicated. Functional status was assessed using the Karnofsky performance score (Ks) both preoperatively and at follow-up visits. Patients’ outcome at 1 year was analyzed against preoperative Ks, tumor grade, tumor size, extent of tumor resection, and access to adjuvant radiotherapy and chemotherapy. Data were analyzed using descriptive and inferential statistics with Chi-square test and the Pearson's contingency coefficient. P ≤ 0.05 was considered statistically significant. Ethical committee approval was obtained for the study.
RESULTS
Glioma was 60 (23.8%) of 252 histology confirmed brain tumors. HGGs represented 32 (53.8% of gliomas) with male:female ratio of 2.2:1.0. Among the HGGs, 40.6% were diagnosed among patients between 31 and 50 years old while 46.8% of cases were 51–70 years old. Glioblastoma multiforme (GBM) accounted for 69% of the HGGs.
Outcome
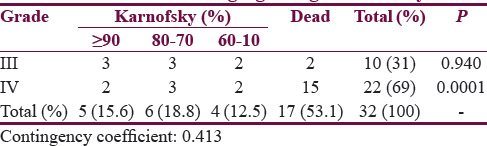
At 1-year postsurgery, 53% of the HGGs were dead and 88% of these deaths were in WHO Grade IV group (contingency coefficient 0.413, P = 0.0001) [Table 1].
Size
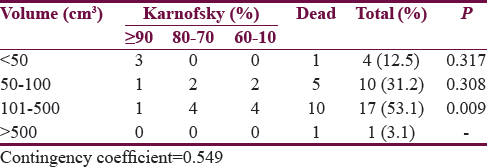
Only four patients had a tumor volume ≤50 cm3, and among these cases, three were independent at 1 year. Tumor size above 101–500 cm3 had the most significant impact on outcome with 58.8% of the patients in this group dead within 1 year (P = 0.009). Patients with tumor volume above 50 cm3 accounted for 94% of the mortality. There was a positive correlation between tumor volume and outcome (contingency coefficient 0.549) [Table 2].
Preoperative Karnofsky
Most of the cases (46.8%) presented with preoperative Ks of 60%, 28% with Ks of 50%, and only 18.9% of these cases presented with preoperative Ks of 70% and above. There was a significant difference in outcome between the cases operated on at a Ks of 60% and below compared with those operated on at Ks of 70% and above (χ2 = 14.496, contingency coefficient = 0.558, P = 0.002) [Table 3]. Based on subgroup analysis, the Ks of 60% and below had more direct impact on mortality with 61.5% of patients operated on at this Ks dying within 1 year (P = 0.0001). This group operated on with Ks ≤60% also represented 94% of mortality at 1 year of all cases operated on (P = 0.002) [Table 3].

Surgery and adjuvant therapy
Gross total resection was achieved in 72% of cases while 15.6% had a subtotal resection and 12.5% had only biopsy, but these did not seem to affect outcome (P = 0.221). Only 40% of cases could receive adjuvant treatment with as low as 15% mortality at 1 year in this subgroup that received adjuvant therapy when compared with 85% of mortality in the group without access to adjuvant treatment (P = 0.005) [Table 4].

DISCUSSION
This study revealed peculiar social and clinical challenges in the management of HGG in the study environment. Late presentation is almost the rule.[112] The late presentation may be indirectly attributed to delay in diagnosis, a relatively poor patient referral system and an uneven spread of imaging and manpower services. Currently, there are about 70 neurosurgeons serving the over 170 million population of Nigeria.[1314] As a result of a small number of neurosurgeons, most of the neurosurgeons reside and work in the urban areas while leaving the rural areas with a poor coverage.
Unfortunately, most primary health care and general practice personnel do not have adequate exposure to most neurosurgery specialty diseases since priority is often given to malaria and other communicable illnesses in training. This coupled with poor access to neuroimaging services outside the urban areas encourages delay in the diagnosis and timely referral of cases for expert care. Although not directly investigated in this study, there are also concerns of social and religious beliefs that may significantly influence patient's attitude toward surgery.[112] The effect of these is that patients are managed at a relatively poor Ks.
More than 80% of the cases were operated at a Ks of 60% and below as a result of delayed presentation. Late surgeries carried out at a low preoperative Ks have been shown to be associated with worse clinical outcomes, including higher morbidity and mortality.[151617] Such cases are also likely to have suboptimal resections, prolonged hospital stay, prolonged ICU stay, and higher cost of management and rehabilitation.[18] As a result of nonavailability of effective national health insurance service (NHIS), this high cost of care is an important consideration in management decision.
In the face of poorer clinical outcomes, the surgeon has to pass through the added challenge of decision to carrying out suboptimal surgery without a guarantee of potential benefit, or leaving the patient with a Ks ≤60% to deteriorate further without surgical intervention. Surgical intervention in this group of patients with relatively poor Ks may still help with management of intracranial pressure, allow opportunity for histology diagnosis, and improve the patient's clinical state and the tumor volume to a level where the patient would be fit for adjuvant radiotherapy and chemotherapy.[101920] These factors have to be discussed carefully with the patient and the relatives. Depending on the anatomical location of the tumor, volume of tumor and tumor relationship to eloquent areas of the brain, gross total resection, should be encouraged as this has been shown to improve patient outcome and effectiveness of adjuvant radiochemotherapy.[101720] This however is not always possible, especially in resource-poor areas where the supportive technology for safe macroscopic excision is not available. Even where such facilities are available, controversy still exists as to the benefits and outcomes following such extensive resections in the poor prognosis varieties of the HGG.
Another challenge of the management of the HGGs identified in this study environment is that a significant proportion of the patients were diagnosed and managed at a relatively young age compared to the Caucasians and most of these cases were diagnosed as Grade IV gliomas. This study observed that as high as 40% of the patients in this study presented for management between 30 and 50 years of age. This young age at diagnosis may suggest a more aggressive disease pattern and needs to be further investigated in the study environment. Secondary GBM is known to be more common among the younger age group and runs a relatively benign course compared to primary GBM.[21] The clinical outcome in the subgroup of relatively younger patients did not bear out this benign course. Since, in general, secondary GBMs are less frequent than the primary GBM,[22] the more aggressive course in the patients managed in the institution of study raises the possibility that more patients managed in the study environment may have de novo GBM despite the relatively young age at diagnosis. This possibility should be explored in future studies.
The extent of resection affects outcome of HGGs. Median survival has been shown to be improved by gross total resection.[20232425] A significant proportion of the cases in this study however presented with big tumor volume and some of these lesions were deep seated in location. As with other studies, these patients operated on with a big tumor volume have more advanced disease, more risk of incomplete excision with a significant residual tumor, higher risk of poorer functional outcome and mortality.[26] These cases will also require longer operation time and intensive care. In addition, when these patients present with alteration of level of consciousness, significant neurological deficits, personality changes, clinical signs of raised intracranial pressure, or signs suggestive of rapid disease progression, the prognosis is likely to be poor despite the extent of resection.[27]
All HGGs need radiotherapy for local control and may require chemotherapy in some patients depending on the DNA methylation status.[2829] Radiotherapy has been demonstrated from other studies to improve long-term survival in patients with HGG, especially among the younger age group.[30] Unfortunately, the access to these adjuvant treatments is still poor in the study environment where also a significant proportion of the patients managed with HGG are in the relatively young age group. This situation may contribute further to worse outcome of these young patients. In the study environment currently, there are only three functional external beam radiotherapy centers serving the radiotherapy needs across the country, resulting in long waiting list. Although this study found a true benefit of adjuvant therapy at 1 year as other studies,[89] the proportion of patients with access to radiotherapy in the study environment was only 40% and some cases could not complete the radiotherapy as a result of breakdown of services.
Attempts have been made to set up the NHIS across the country, but this has remained less than ideal. High-earned specialties such as neurosurgery are also poorly represented in the NHIS. This has added the challenge of out-of-pocket payment for these medical services. This also makes the access to experimental and other evolving therapeutic modalities such as intracavitary Gliadel, immunotherapy, biologic response modifiers, oncolytic viruses, and gene therapy still luxury in the study environment.
These social and clinical factors highlighted in this study have contributed indirectly to prolonged ICU stay, high financial burden for treatment, and more morbidity and mortality. Addressing these challenges including improvement in the national health insurance scheme for the masses will go a long way on the long run.
CONCLUSION
HGGs are quite common in Nigeria, and the rate of diagnosis is increasing. The peak age at diagnosis of the HGGs seem to be lower than in the Caucasians with higher proportions of these lesions being GBM. Most cases present late with a poor Ks and big tumor volume. The proportion of patients with access to adjuvant treatment is still poor. Addressing these local challenges including adequate coverage of the NHIS may improve the disease outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Symptomatic primary intracranial neoplasms in Nigeria, West Africa. J Neurol Sci (Turk). 2007;24:212-8.
- [Google Scholar]
- Spectrum of intracranial tumours in a tertiary health carefacility: Our findings. Pan Afr Med J. 2015;20:24.
- [Google Scholar]
- Quality of life in patients with intracranial gliomas: The impact of modern image-guided surgery. J Neurosurg. 2011;114:1622-30.
- [Google Scholar]
- Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21:201-6.
- [Google Scholar]
- Combined surgery, radiation, and PCV chemotherapy for astrocytomas compared to oligodendrogliomas and oligoastrocytomas WHO grade III. J Neurooncol. 2002;59:231-7.
- [Google Scholar]
- Effectiveness of radiotherapy for elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81:206-10.
- [Google Scholar]
- Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-96.
- [Google Scholar]
- Supratentorial gliomas: Surgical considerations and immediate postoperative results. Gross total resection versus partial resection. Neurosurgery. 1987;21:21-6.
- [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO Classification of Tumors of the Central Nervous System. World Health Organisation Classification of Tumours (4th ed). Lyon: International Agency for Research on Cancer; 2007. p. :309.
- Epilepsy in primary intracranial tumors in a neurosurgical hospital in Enugu, South-East Nigeria. Niger J Clin Pract. 2015;18:681-6.
- [Google Scholar]
- Neurosurgery in Nigeria – An evaluation of the perception of health personnel in a new centre and a comparison of the Nigerian situation with that of other African states. Niger J Clin Pract. 2008;11:291-5.
- [Google Scholar]
- National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989;71:826-36.
- [Google Scholar]
- Prognostic factors for survival of patients with glioblastoma: Recursive partitioning analysis. Neuro Oncol. 2004;6:227-35.
- [Google Scholar]
- Prognostic factors for high grade glioma: Development of a prognostic index. J Neurooncol. 1990;9:47-55.
- [Google Scholar]
- The role of cytotoxic chemotherapy in the treatment of malignant brain tumors. Surg Neurol. 1995;44:551-2.
- [Google Scholar]
- Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753-64.
- [Google Scholar]
- The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764-72.
- [Google Scholar]
- Astrocytomas. In: Greenberg MS, ed. Handbook of Neurosurgery (8th ed). New York: Thieme Medical Publishers; 2016. p. :612-28.
- [Google Scholar]
- The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371-9.
- [Google Scholar]
- Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery. 2008;62:564-76.
- [Google Scholar]
- A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-8.
- [Google Scholar]
- The prognostic importance of tumor size in malignant gliomas: A computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol. 1988;6:338-43.
- [Google Scholar]
- Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61:665-73.
- [Google Scholar]
- Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011-8.
- [Google Scholar]
- Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350-4.
- [Google Scholar]
- Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333-43.
- [Google Scholar]






