Translate this page into:
Stroke in Tuberculous Meningitis and Its correlation with Magnetic Resonance Angiography Manifestations
Abhay Ranjan, MD, DM, PDF Department of Neurology, Indira Gandhi Institute of Medical Sciences Patna 800014, Bihar India drabhayranjan97@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objective The primary objective of the study was to assess the location of cerebral infarction and look for corresponding magnetic resonance angiography (MRA) changes in patients with tuberculous meningitis (TBM). We also evaluated the predictors of ischemic stroke in TBM and the impact of these infarctions on patient's outcome.
Methods This was a single-center prospective study between September 2018 and September 2020. Demographic and laboratory parameters were noted. Cranial magnetic resonance imaging and MRA were performed at the time of admission to the hospital.
Results Among 120 patients with TBM, 46 had stroke. Nineteen (15.8%) patients died, of which 12 (10%) suffered from stroke. The most common site of infarction was the basal ganglia (54.3%). The commonest site of MRA abnormalities was the middle cerebral artery (39.1%). British Medical Research Council (BMRC) stage 3, cerebrospinal fluid (CSF) sugar, CSF adenosine deaminase (ADA) level, basal exudates, hydrocephalus, and hyponatremia were found to be predictors of stroke in TBM, while BMRC stage 3, CSF cell count, CSF ADA level, and anemia were found to be significantly associated with mortality in TBM patients with stroke.
Conclusion The basal ganglia were the most common site of ischemic stroke in TBM, and middle cerebral artery was the most often involved intracranial blood vessel. BMRC stage 3 was significantly associated with both stroke and mortality in TBM patients with stroke.
Keywords
tuberculous meningitis
stroke
infarction
MR angiography
predictors
mortality
Introduction
Tuberculous meningitis (TBM) is a serious form of extrapulmonary tuberculosis (TB) and constitutes around 10% of all TB cases.1 Severe complications of TBM include stroke, hydrocephalus, and tuberculoma formation. In developing countries, 40% of the mortality due to TB is caused by TBM. Mortality due to TBM in India is around 1.5/100,000 population.2
About two-third of TBM patients may develop stroke during illness.3 There are different data on the frequency of infarcts in autopsy and imaging-based studies because neuroimaging is less sensitive for detecting small infarcts in the brainstem.4 The mortality rate of TBM patients with stroke is three times higher, and cerebral infarction is the main determinant of disability in survivors.5 Other predictors of mortality in TBM are age, higher stage, human immunodeficiency virus (HIV) infection, and hydrocephalus.6 7
Abnormalities in magnetic resonance angiography (MRA) are found in around 50% of TBM patients.8 9 Compared with normal MRA, abnormal MRA at baseline can predict stroke at follow-up.8 There are few studies in the literature on the changes of MRA in patients with TBM and stroke.
The purpose of this study was to evaluate the location of cerebral infarction in patients with TBM and to find the corresponding changes in MRA. We also evaluated the predictors of ischemic stroke in TBM and the impact of these infarcts on the outcome of patients.
Subject and Methods
Study Design and Participants
This was a single-center prospective study and we recruited TBM patients who were admitted to the Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India between September 2018 to September 2020. The study was approved by the institutional ethics committee. Written informed consent to participate in the study was obtained from all patients or their guardians.
Inclusion
Patients fulfilling the following inclusion criteria were included: (1) age 12 years and above, (2) written informed consent, and (3) TBM diagnosis based on Lancet scoring system published in 2010.10 Patients were defined as definite TBM when acid-fast bacilli were seen in the cerebrospinal fluid (CSF) or if CSF was positive for commercial nucleic acid amplification test. Probable TBM was diagnosed if their diagnostic score was ≥ 12 (at least 2 points either come from CSF or cerebral imaging criteria) plus exclusion of alternative diagnoses. Possible TBM was diagnosed if their score was between 6 and 11 points.10
Exclusion
TBM patients with vascular risk factors such as the history of hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, atrial fibrillation, ischemic stroke, and intracerebral hemorrhage were excluded.
Evaluation
Detailed clinical history including demographic details of TBM patients was obtained. Patients were clinically evaluated for signs of meningeal irritation, raised intracranial pressure, cranial nerve deficits, focal neurological deficits, and evidence of extraneural TB. Glasgow Coma Scale (GCS) was used to assess the level of consciousness. Clinical severity at admission was achieved using British Medical Research Council (BMRC) staging, that is, stages 1, 2, and 3.1 The clinical outcome was assessed by survival or in-hospital death.
Investigation
Patients underwent investigations that included routine blood counts, serum biochemistry, erythrocyte sedimentation rate, HIV serology, and chest radiograph. CSF was examined for opening pressure, cells (lymphocyte and neutrophil differential count), glucose, protein, acid-fast bacilli smear, culture, gene expert nucleic acid amplification test. India ink preparation was performed to exclude cryptococcal meningitis.
Brain MRI was performed on 1.5 T (Optima MR 360, General Electric Medical Systems, Wisconsin, United States) magnetic resonance imaging (MRI) system incorporating whole-brain axial fluid-attenuated inversion recovery, diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC), time-of-flight MRA in addition to T2-weighted and pre- and postgadolinium T1 weighted sequence. The patients were assessed for leptomeningeal enhancement, tuberculoma, cerebral infarction, hydrocephalus, ventriculitis, cranial nerve involvement, and significant stenosis in major cerebral arteries. Cerebral infarctions were recorded according to location and number. Stroke was diagnosed on the basis of DWI hyperintensity and corresponding hypointensity on ADC. MR angiographic abnormalities were characterized by greater than 50% stenosis of short or long segments of intracranial arteries.11
Treatment
All patients were prescribed four drug-intensive anti-TB therapies (rifampicin, isoniazid, pyrazinamide, ethambutol) with pyridoxine and steroid (dexamethasone)according to recommendations of the World Health Organization.12
Statistical Analyses
Statistical analyses were performed using Statistical Package for Social Sciences, SPSS (Version 20.0, SPSS Inc.) software. The categorical variable was compared using the chi-squared test (or Fisher's exact test) and continuous variables were compared using the independent t-test. Predictors of mortality were assessed using binary logistic regression analysis, where the variable had a p-value < 0.1 in univariate analysis. Variable with two-tailed p-values ≤ 0.05 was considered significant.
Results
Baseline Characteristics
In this study, 134 patients fulfilled the criteria for the diagnosis of TBM. Of these 134 patients, 14 patients were excluded, that is, 6 patients had hypertension, 3 had diabetes, 1 had both hypertension and diabetes, 2 had valvular heart disease with atrial fibrillation, and 2 had dyslipidemia (Fig. 1).

-
Fig. 1 Description of inclusion and exclusion of tuberculous meningitis (TBM) patient in the study.
Fig. 1 Description of inclusion and exclusion of tuberculous meningitis (TBM) patient in the study.
One-hundred twenty TBM patients were enrolled in the study, of which 46 patients had stroke. The median age was 26 years (13–70 years), of which 45.8% were women. About 26.7% of patients were defined as definite TBM, 45.8% as probable TBM, and 27.5% as probable TBM. MRI abnormalities were found in 95.8% of patients, including hydrocephalus in 39.2%, infarction in 38.3%, and tuberculoma in 37.5%.
According to the location of the infarction in the stroke group, there were 25 cases of basal ganglia infarction (54.3%), 20 cases of cortical infarction (43.5%), 16 cases of thalamic infarction (34.8%), 9 cases of brainstem infarction (19.6%), and 3 cases of cerebellar infarction (6.5%). Multifocal infarcts occurred in 37% of patients.
Comparison of Clinical, Laboratory, and Imaging Parameters between Stroke and Nonstroke Patients in TBM
The median age of patients in the stroke group was 25.5 years, compared with 26.5 years in the nonstroke group (p = 0.338). There were significant differences in mean GCS of the patients in the stroke group (11.3 ± 3.1) compared with the nonstroke group (13.22 ± 2.5). There were significant differences in mean serum sodium level with low serum sodium level in the stroke group as compared with the nonstroke group (130.83 ± 6.6 vs. 134 ± 6.6, p = 0.003). CSF examination revealed significantly lower blood glucose levels and higher adenosine deaminase (ADA) in stroke patients (32 mg/dL vs. 43.5 mg/dL, p = 0.002, 32 U/L vs. 24 U/L, p = 0.017, respectively) (Table 1).
|
Total (n = 120) |
Infarct (n = 46) |
Noninfarct (n = 74) |
p-Value |
|
|---|---|---|---|---|
|
Age (years) Median (range) |
26 (5–70)] |
25.5 (9–65) |
26.5 (5–70) |
0.338 |
|
Sex (male, %) |
54.2 (65) |
60.9 (28) |
50 (37) |
0.165 |
|
Duration of stay (days) Median (range) |
12 (2–50) |
14 (2–50) |
11 (2–41) |
0.091 |
|
Fever (days) Median (range) |
30 (5–180) |
45 (5–180) |
30 (5–180) |
0.486 |
|
Headache (days) Median (range) |
30 (2–365) |
30 (5–365) |
30 (2–365) |
0.289 |
|
Vomiting (days) Median (range) |
20 (2–150) |
25 (5–120) |
15 (2–150) |
0.590 |
|
Seizure, % (n) |
29.2 (35) |
34.8 (16) |
25.7 (19) |
0.194 |
|
Hemiparesis, % (n) |
14.2(17) |
30.4 (14) |
4.1% (3) |
|
|
Cranial nerve, % (n) |
29.2 (35) |
21.7 (10) |
33.8 (25) |
0.113 |
|
GCS score |
12.45 (4–15) |
11.33 ± 3.1 |
13.22 + 2.5 |
0.0001 |
|
BMRC stage |
||||
|
Stage 1, % (n) |
28.3 (34) |
13 (6) |
37.8 (28) |
0.003 |
|
Stage 2, % (n) |
45 (54) |
43.5 (20) |
45.2 (34) |
0.794 |
|
Stage 3, % (n) |
26.7 (32) |
43.5 (20) |
16.2 (12) |
0.001 |
|
Type of TBM |
||||
|
Definite TBM, % (n) |
26.7 (32) |
28.3 (13) |
25.7 (19) |
0.758 |
|
Probable TBM, % (n) |
45.8 (55) |
63 (29) |
35.1 (26) |
0.003 |
|
Laboratory findings |
||||
|
Hemoglobin (mg/dL) Mean (SD) |
11.88 (1.89) |
11.63 (2.09) |
12.03 (1.75) |
0.267 |
|
TLC (/cumm) mean (SD) |
8135.4 (3366) |
8236(3508) |
8072 (3297) |
0.80 |
|
SGOT(U/L) mean (SD) |
51.61 (92.11) |
59.57 (137.65) |
46.66 (45.67) |
0.45 |
|
SGPT(U/L) mean (SD) |
48.51 (84) |
57.65 (131.5) |
42.82 (27.5) |
0.45 |
|
Serum albumin gm/dL Mean (SD) |
3.89 (0.62) |
3.85 (0.729) |
3.92 (0.54) |
0.54 |
|
Hyponatremia (serum sodium < 135 mmol/L) % (n) |
60 (72) |
71.7 (33) |
52.7 (39) |
0.029 |
|
CSF findings |
||||
|
CSF protein (mg/dL) Median (range) |
175 (16–4902) |
196 (55–4902) |
155 (16–3110) |
0.598 |
|
CSF sugar mg/dL Median (range) |
34.4 (7–101) |
32 (8–65) |
43.5 (7–101) |
0.002 |
|
CSF total count /cumm Median (range) |
72 (2–1100) |
95 (2–640) |
50 (4–1100) |
0.113 |
|
CSF ADA U/L Median (range) |
25 (2–98) |
32 (4–98) |
24(2–89) |
0.017 |
|
Imaging finding |
||||
|
Basal exudate, % (n) |
38.3 (46) |
54.3 (25) |
28.4 (21) |
0.004 |
|
Hydrocephalus, % (n) |
39.2 (47) |
52.2 (24) |
31.1 (23) |
0.018 |
|
Meningeal enhance, % (n) |
75.8 (91) |
80.4 (37) |
73 (54) |
0.241 |
|
Tuberculoma, % (n) |
37.5 (45) |
37 (17) |
37.8 (28) |
0.54 |
|
Mortality, % (n) |
15.8 (19) |
26.1 (12) |
9.5 (7) |
0.016 |
Abbreviations: ADA, adenosine deaminase; BMRC, British Medical Research Council; CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale; SD, standard deviation; SGOT, serum glutamic oxaloacetic transminase; SGPT, serum glutamic pyruvic transaminase; TBM, tuberculous meningitis; TLC, total leucocyte count.
On MRI, patients having basal exudates and hydrocephalus were significantly more in stroke patients than nonstroke patients (52.2 vs. 31.1%, p = 0.018, 54.3 vs. 28.4%, p = 0.004, respectively). Stroke patients were significantly higher in stage 3 TBM compared with nonstroke patients (43.5 vs. 16.2%, respectively, p = 0.001).
Cerebral MRA showed abnormalities in 30 (65.2%) patients with stroke. Single arterial abnormalities were seen in 47.8% (n = 22) patients and multiple arterial involvement in 17.4% (n = 8) patients. Anterior circulation was involved in 52.2% (n = 24) and posterior circulation involvement was present in 13% (n = 6) of patients. The M1 segment of the middle cerebral artery (MCA) was the most frequently involved artery at 39.1% (n = 18), followed by the anterior cerebral artery (ACA) at 21.7% (n = 10), the internal carotid artery (ICA) at 17.4% (n = 8), and the posterior cerebral artery (PCA) at 13% (n = 6) patients (Figs. 2,3,4). In 16 patients of TBM with stroke, no MRA abnormalities were observed.
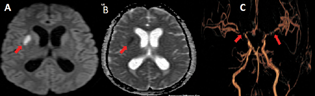
-
Fig. 2 A 23-year-old male with tuberculous meningitis (altered sensorium and left sided weakness). (A) Axial diffusion-weighted imaging (arrow) and (B) axial apparent diffusion coefficient (arrow) show acute infarct in right putamen. (C). Volume-rendered magnetic resonance imaging (arrows) demonstrates severe stenosis of supraclinoid and terminal segment of right internal carotid artery (ICA), cavernous and terminal segment of left ICA, M1 segment of bilateral middle cerebral artery, and A1 segment of bilateral anterior cerebral artery. Hydrocephalus can also be seen.
Fig. 2 A 23-year-old male with tuberculous meningitis (altered sensorium and left sided weakness). (A) Axial diffusion-weighted imaging (arrow) and (B) axial apparent diffusion coefficient (arrow) show acute infarct in right putamen. (C). Volume-rendered magnetic resonance imaging (arrows) demonstrates severe stenosis of supraclinoid and terminal segment of right internal carotid artery (ICA), cavernous and terminal segment of left ICA, M1 segment of bilateral middle cerebral artery, and A1 segment of bilateral anterior cerebral artery. Hydrocephalus can also be seen.
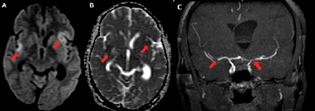
-
Fig. 3 A 27-year-old female with tuberculous meningitis. (A) Axial diffusion-weighted imaging (arrow) and (B) axial apparent diffusion coefficient (arrow) demonstrate acute infarct in bilateral sylvian cortex with gyriform diffusion restriction. (C) Coronal maximum intensity projection magnetic resonance imaging (arrow) shows severe stenosis of bilateral M1 segment of middle cerebral artery. Asymmetrically dilated lateral ventricles can also be seen.
Fig. 3 A 27-year-old female with tuberculous meningitis. (A) Axial diffusion-weighted imaging (arrow) and (B) axial apparent diffusion coefficient (arrow) demonstrate acute infarct in bilateral sylvian cortex with gyriform diffusion restriction. (C) Coronal maximum intensity projection magnetic resonance imaging (arrow) shows severe stenosis of bilateral M1 segment of middle cerebral artery. Asymmetrically dilated lateral ventricles can also be seen.
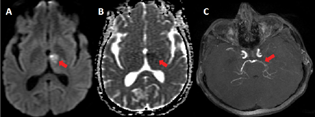
-
Fig. 4 A 18-year-old girl with multidrug resistant tuberculous meningitis. (A) Axial diffusion-weighted imaging (arrow) and (B) axial apparent diffusion coefficient (arrow) show acute infarct in left thalamus. (C) Axial maximum intensity projection magnetic resonance angiography image (arrow) reveals marked stenosis in left P1and P2 segment of posterior cerebral artery.
Fig. 4 A 18-year-old girl with multidrug resistant tuberculous meningitis. (A) Axial diffusion-weighted imaging (arrow) and (B) axial apparent diffusion coefficient (arrow) show acute infarct in left thalamus. (C) Axial maximum intensity projection magnetic resonance angiography image (arrow) reveals marked stenosis in left P1and P2 segment of posterior cerebral artery.
Predictors of Stroke in TBM
GCS at presentation, BMRC stage 3, CSF sugar, CSF ADA level, basal exudates, hydrocephalus, and probable TBM were found to be independent risk factors for stroke in TBM (Table 2).
|
Variables |
Value (n = 46) |
p-Value |
|---|---|---|
|
BMRC stage 3, % (n) |
43.5% (n = 20) |
0.001 |
|
CSF sugar median (range) |
32 (8–65) mg/dL |
0.002 |
|
CSF ADA median (range) |
32 (4–98) mg/dL |
0.017 |
|
Hydrocephalus, % (n) |
52.2% (n = 24) |
0.018 |
|
Basal exudate, % (n) |
53.34% (n = 25) |
0.004 |
|
Hyponatremia, % (n) |
71.7% (n = 33) |
0.029 |
Abbreviations: ADA, adenosine deaminase; BMRC, British Medical Research Council; CSF, cerebrospinal fluid; TBM, tuberculous meningitis.
Outcome Assessment
Nineteen (15.8%) patients in the study population died, of which 12 (10%) suffered from stroke. On univariate analysis, stroke (26.1 vs. 9.5%, p = 0.016), basal exudates (p = 0.05), hydrocephalus (p = 0.004), BMRC stage 3 (p = 0.00), raised CSF protein (p = 0.001), raised CSF total cell count (p = 0.00), low CSF glucose (p = 0.014), increased CSF ADA (p = 0.001), and decreased hemoglobin (p = 0.004) were found to be significantly associated with mortality in TBM patients, but on multivariate binary logistic regression analysis, raised CSF total cell count (p = 0.024), increased CSF ADA (p = 0.025), and decreased serum hemoglobin (p = 0.04) were found to be an independent predictor of mortality in patients with TBM (both stroke and nonstroke). Stroke was not found to be an independent risk factor for death in TBM patients (p = 0.63).
In univariate analysis, predictors of mortality in the stroke group were CSF cell count (increased cell count), CSF ADA level, anemia, and BMRC stage 3 (Table 3). In multivariate binary logistic regression analysis, increased CSF cell count and BMRC stage 3 were found to be predictors of mortality in stroke patients. No significant statistical correlation was observed when comparing the presence of MR angiographic changes (p = 0.570) and location of the artery involved (MCA p = 0.839, ACA p = 0.199, PCA= 0.58, ICA p = 0.94) with mortality in TBM patients with stroke.
|
Variables |
Mortality (n = 12) |
p-Value |
|---|---|---|
|
BMRC stage 3, % (n) |
75% (n = 9) |
0.013 |
|
CSF total count/cumm Mean (SD) |
301.58 (195.23) |
0.001 |
|
CSF ADA U/L Mean (SD) |
47.83 (28.88) |
0.037 |
|
Anemia mg/dL Mean (SD) |
10.58(2.7) |
0.028 |
Abbreviations: ADA, adenosine deaminase; BMRC, British Medical Research Council; CSF, cerebrospinal fluid; SD, standard deviation; TBM, tuberculous meningitis.
Discussion
In our study, 46 (38.3%) TBM patients were found to have infarcts that are comparable to previous studies done by Chan et al and Kalita et al.13 14 Several mechanisms have been postulated for infarction in TBM patients. The most commonly accepted mechanism of infarction is the presence of basal exudates, which leads to vasculitis, thrombosis, vasospasm, and vasoconstriction across the base of the skull.5 Hypoperfusion due to hypovolemia caused by cerebral salt wasting syndrome leads to hyponatremia, which plays a role in the pathogenesis of stroke in TBM.15 Hydrocephalus in TBM leads to stretching of blood vessels and decreased cerebral blood flow leading to cerebral ischemia.5
Basal meningeal enhancement and CSF cell count are found to be risk factors for stroke in TBM patients, showing role of the inflammation in pathogenesis of stroke in TBM,16 although vascular risk factors, like age, hypertension, diabetes mellitus, and dyslipidemia, were found to be determinant of stroke in TBM in other studies.17 The etiology of stroke differs in young and elderly. Atherosclerosis of the large arteries is the main cause of stroke in the elderly, but it accounts for only ∼10 to 15% of strokes in young people. Cardiac embolism, extracranial artery dissection, infection-related vasculitis, migraine, and drug use are the main causes of stroke in young people.18 We had excluded vascular risk factors from our study to mitigate the effects of atherosclerosis. The contribution of atherosclerosis on stroke in patients with TBM is still controversial because it mainly involves perforator and terminal cortical branches rather than the main intracranial arteries seen in neuroimaging.3 Vasculitis and intimal proliferation were found in histopathological studies, but not thrombosis in patients of TBM.19
In our study, the basal ganglia were the most common site of infarction (54.3%), which was comparable to previous studies by Tai et al (49%), Kalita et al, (54%) and Zhang et al (50%).3 14 16 Tai et al observed that cerebral infarction in TBM involved mainly the perforators and terminal cortical branches, so vascular supply classification has to be used rather than “Tubercular zone” versus “ischemic zone.”3
We observed that in this study, 65.2% of TBM patients with stroke had angiographic changes suggestive of vasculitis. Large intracranial arteries at the base of the brain were involved similar to previous studies.5 8 The commonest site of MRA abnormalities in our study was MCA (39.1%). Other studies have also shown that MCA is the most common vessel involved in MRA.8 9 In our study, approximately one-third (34.8%) of patients had an infarction, but MRA was not abnormal. A study by Gupta et al also showed no corresponding MRA changes in one-third of patients.20 In a study by Kalita et al, ∼50% of the patients with infarction did not have corresponding MRA abnormalities. This may be due to the limitations of MRA in delineating small-caliber vessels, recanalization, or other immune-mediated vascular damage that lead to infarction.8
This study showed that stage of TBM, CSF sugar, CSF ADA, basal exudates, hydrocephalus, and probable TBM were independent risk factors for acute stroke in TBM. The stage of TBM was also found to be a predictor of stroke in previous studies from India.14 21 22 Similar to our study, previous studies have also shown an association of neuroimaging findings, that is, hydrocephalus and basal exudates as predictors of stroke.14 23 We also observed that CSF glucose and serum sodium were significantly low in patients with stroke compared with nonstroke patient. Previous studies have revealed a significant association between CSF cell count and stroke in TBM, but in our study, although there was an increase in CSF cell count in stroke patients, it was not statistically significant.16 23
Mortality in TBM patients with stroke was approximately three times higher in our study compared with nonstroke patients, which is comparable to previous studies.5 13 14 17 22 In this study, the independent predictors of mortality in the stroke group were BMRC stage 3, CSF cell count, CSF ADA, and anemia. On multiple logistic regression, total CSF cell count and BMRC stage 3 were found to be a significant predictor of mortality in stroke patient. BMRC stage 3, anemia, and increasing CSF ADA were associated with increased mortality in TBM patients.6 7 24
We did not find any association between mortality and MRA changes, as seen in previous studies by Lu et al and Kalita et al.9 14
Conclusion
In the present study, we found that ischemic stroke is common in TBM, affecting about one-third of patients. Stage 3 of TBM, CSF sugar, CSF ADA, basal exudates, and hydrocephalus are predictors for stroke in TBM. The outcome of stroke patients was worse compared with nonstroke patients. About two-third of TBM patients with stroke have MRA abnormalities, with MCA being the most common.
Conflict of Interest
None declared.
Funding None.
References
- Cerebral infarction and tuberculoma in central nervous system tuberculosis: frequency and prognostic implications. J Neurol Neurosurg Psychiatry. 2014;85(11):1260-1264.
- [Google Scholar]
- Estimating mortality from tuberculous meningitis in a community: use of available epidemiological parameters in Indian context. Indian J Tuberc. 2000;47:9-12.
- [Google Scholar]
- Vascular complications of tuberculous meningitis: an autopsy study. Neurol India. 2015;63(6):926-932.
- [Google Scholar]
- Mortality in hospitalized patients with tuberculous meningitis. BMC Infect Dis. 2019;19(1):9.
- [Google Scholar]
- Derivation of a bedside score (MASH-P) to predict 6-month mortality in tuberculous meningitis. J Neurol Sci. 2020;415:116877.
- [Google Scholar]
- Magnetic resonance angiography manifestations and prognostic significance in HIV-negative tuberculosis meningitis. Int J Tuberc Lung Dis. 2015;19(12):1448-1454.
- [Google Scholar]
- Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803-812.
- [Google Scholar]
- A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643-646.
- [Google Scholar]
- Treatment of Tuberculosis: Guidelines. (4th edition.). Geneva: World Health Organisation; 2010.
- [Google Scholar]
- Cerebral infarcts complicating tuberculous meningitis. Cerebrovasc Dis. 2005;19(6):391-395.
- [Google Scholar]
- Predictors of stroke and its significance in the outcome of tuberculous meningitis. J Stroke Cerebrovasc Dis. 2009;18(4):251-258.
- [Google Scholar]
- Mechanism, spectrum, consequences and management of hyponatremia in tuberculous meningitis. Wellcome Open Res. 2021;4:189.
- [Google Scholar]
- Acute ischemic stroke in young adults with tuberculous meningitis. BMC Infect Dis. 2019;19(1):362.
- [Google Scholar]
- Frequency and impact of cerebral infarctions in patients with tuberculous meningitis. Stroke. 2018;49(10):2288-2293.
- [Google Scholar]
- MR imaging and angiography in tuberculous meningitis. Neuroradiology. 1994;36(2):87-92.
- [Google Scholar]
- Predictors of stroke in patients of tuberculous meningitis and its effect on the outcome. QJM. 2010;103(9):671-678.
- [Google Scholar]
- Identification of predictors of cerebrovascular infarcts in patients with tuberculous meningitis. Int J Mycobacteriol. 2020;9(3):303-308.
- [Google Scholar]
- Clinical characteristics and treatment delay of cerebral infarction in tuberculous meningitis. Intern Med J. 2012;42(3):294-300.
- [Google Scholar]
- Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351(17):1741-1751.
- [Google Scholar]







