Translate this page into:
Staged Multimodality Treatment of a Large Ruptured Fusiform Supraclinoid Internal Carotid Artery Aneurysm: Microsurgical Clip-assisted Endovascular Coiling
Address for correspondence: Dr. Dale Ding, Department of Neurosurgery, Barrow Neurological Institute, P. O. Box: 800212, Charlottesville, VA 22908, USA. E-mail: daleding1234@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the contemporary era of aneurysm management, large fusiform aneurysms presenting with subarachnoid hemorrhage (SAH) remain particularly challenging lesions to successfully manage. We describe a staged, multimodal treatment strategy for a 71-year-old patient who presented with a large ruptured fusiform aneurysm of the supraclinoid internal carotid artery (ICA) and a fetal posterior communicating artery which originated from the inferomedial aspect of the aneurysm. In the first stage, we performed a partial microsurgical clip reconstruction of the fusiform aneurysm and secured its rupture site, which was identified intraoperatively. This left two residual saccular components of the aneurysm, which were targeted with endovascular coiling in the same hospitalization after the patient had convalesced from the SAH and was beyond the vasospasm window. We believe that this combined approach of clip-assisted coiling can be employed instead of endovascular flow diversion or microsurgical bypass for appropriately selected patients with ruptured fusiform ICA aneurysms.
Keywords
Endovascular procedures
fusiform
intracranial aneurysm
microsurgery
subarachnoid hemorrhage
INTRODUCTION
Intracranial aneurysms infrequently are infrequently fusiform in morphology. Unlike saccular aneurysms, which are anatomically distinct from the parent artery, fusiform lesions circumferentially involve a diseased segment of the parent vessel.[123] Despite recent advances in the microsurgical and endovascular technologies for the treatment of intracranial aneurysms, fusiform aneurysms continue to pose unique and considerable challenges to modern cerebrovascular surgeons.[45] The management options are particularly limited for fusiform aneurysms presenting with subarachnoid hemorrhage (SAH), large lesions, and those in patients without insufficient collateral supply of the vulnerable brain region. In this case report, we describe our technique for the treatment of a large, ruptured fusiform aneurysm of the supraclinoid internal carotid artery (ICA) using a staged, multimodality approach.
CASE REPORT
A 71-year-old female, who had previously undergone a craniotomy for clipping of a ruptured left posterior communicating artery (PCOM) aneurysm 18 years prior, presented with a Hunt and Hess Grade II, Fisher Grade III SAH. Computed tomography angiography (CTA) and catheter cerebral angiography showed a large (12.5 mm × 13.3 mm), fusiform supraclinoid ICA aneurysm arising around the previous clip and extending to the ICA bifurcation [Figure 1]. The patient had a fetal left PCOM, which arose from the inferomedial portion of the aneurysm. After a multidisciplinary discussion regarding the treatment options, we decided to proceed with a staged multimodality approach, including partial microsurgical clip reconstruction (Stage 1), followed by endovascular embolization of the residual aneurysm (Stage 2).
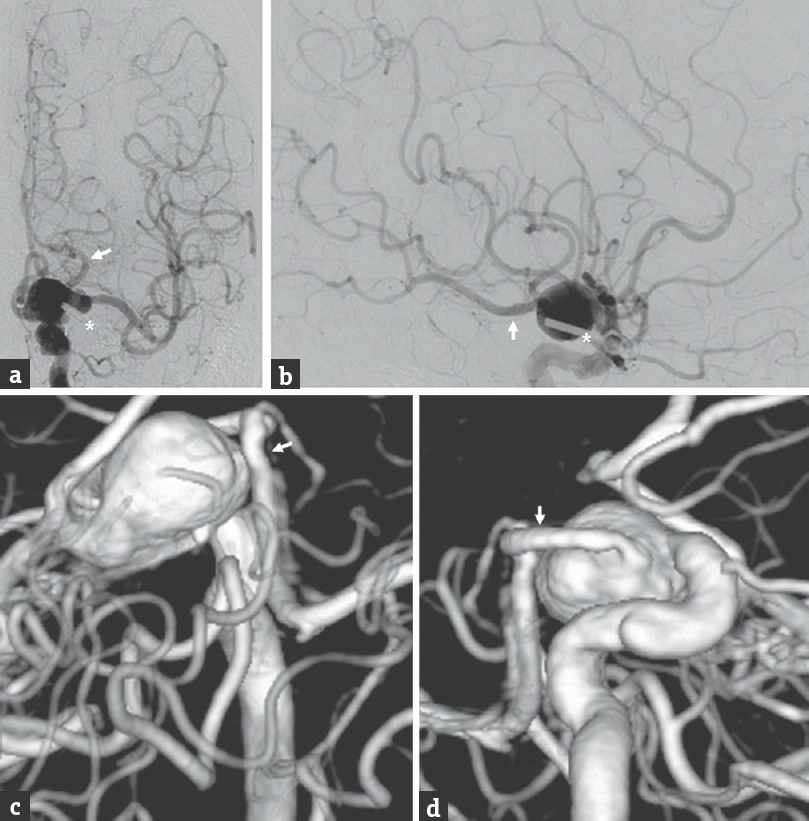
- Endovascular procedures, fusiform, intracranial aneurysm, microsurgery, subarachnoid hemorrhage preoperative cerebral angiography, (a) anteroposterior and (b) lateral views of a left internal carotid artery injection, shows a fusiform aneurysm of the left supraclinoid internal carotid artery (predominantly C7 segment) which incorporates the fetal posterior communicating artery origin and extends to the internal carotid artery terminus. The in situ clip used to treat patient's previously ruptured posterior communicating artery aneurysm can be visualized on the subtracted angiogram (asterisk). Preoperative three-dimensional reconstruction of a computed tomography angiography, with views from (c) above and (d) medially. The left fetal posterior communicating artery can be seen arising from the medial aspect of the fusiform aneurysm in the surgical blind spot (arrow)
The patient was positioned in a standard fashion for a pterional craniotomy, except with slightly less neck extension to allow for a better surgical view of the anterior clinoid process (ACP). We reopened the patient's prior pterional craniotomy and extended it medially to expose a greater extent of the basal frontal lobe. We used the subfrontal corridor to expose the ipsilateral optic nerve and ICA. The previous clip was identified lateral to the ipsilateral ACP. We then opened the Sylvian fissure from a distal to proximal direction to identify the M1 and M2 segments of the middle cerebral artery. Next, the basal cisterns around the left optic nerve and ICA were opened to expose the fusiform aneurysm and ICA bifurcation. On further dissection of the aneurysm, we identified a superomedial area which appeared to be the site of rupture.
Although the entirety of the fusiform aneurysm could be visualized at this point, it was too bulbous for clipping without proximal control. Therefore, we performed an intradural anterior clinoidectomy in a standard fashion. We then incised the lateral distal dural ring, which exposed the C5 (clinoidal) segment of the ICA and resected the falciform ligament overlying the left optic nerve. This allowed us to place a temporary clip on the clinoidal ICA, which sufficient softened the fusiform aneurysm to allow for clip reconstruction. Two fenestrated clips were used to reconstruct the distal portion of the aneurysm (between the PCOM and ICA bifurcation) and secure the rupture site.
The temporary clip was removed, and the clip from the patient's previous surgery was dissected from the roof of the cavernous sinus and removed. This revealed the fetal PCOM arising from the medial aspect of the aneurysm. After reapplying the temporary clip, we used another two fenestrated clips to reconstruct the proximal portion of the aneurysm (below the PCOM). Postoperative CTA and cerebral angiography showed two residual aneurysm components, including a superior component arising from above the clip construct and an inferior component arising between the superior and inferior sets of two fenestrated clips [Figure 2]. The fetal PCOM originated from the inferior component.
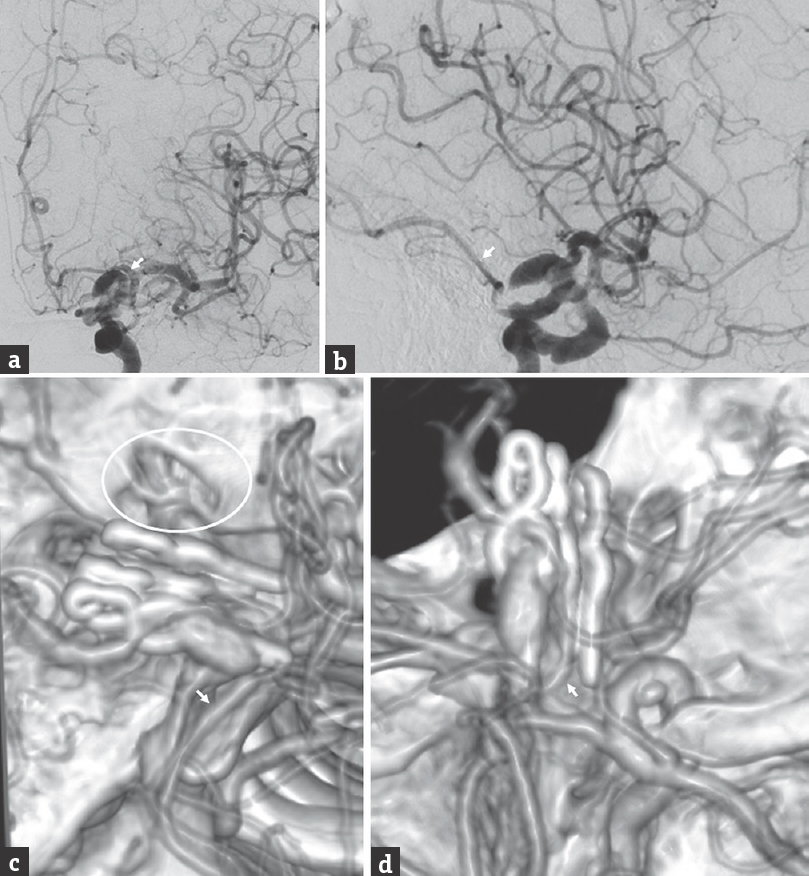
- Postoperative cerebral angiography performed after craniotomy for partial clip reconstruction of the ruptured fusiform left internal carotid artery aneurysm, (a) anteroposterior and (b) lateral views of a left internal carotid artery injection, and postoperative three-dimensional reconstruction of a computed tomography angiography, with views from (c) above and (d) medially. The residual aneurysm has two saccular components, and the left fetal posterior communicating artery (arrow) originates from the inferior component. We performed an intradural anterior clinoidectomy (circle), which allowed proximal control of the fusiform aneurysm through exposure of the C5 (clinoidal) segment of the internal carotid artery
The patient subsequently underwent endovascular treatment of clinical vasospasm and ventriculoperitoneal shunt placement for post-SAH hydrocephalus. After the patient had endured the sequelae of her SAH and was beyond the vasospasm window, we performed endovascular coiling of both residual aneurysm components during the same hospitalization [Figure 3]. The remainder of her hospital course was uneventful, and she was discharged to a rehabilitation facility in neurologically stable condition.
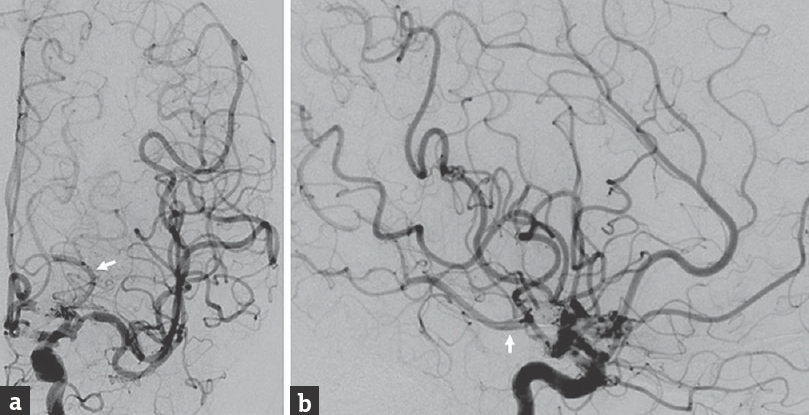
- Postembolization cerebral angiography performed after coiling of the residual fusiform internal carotid artery aneurysm, (a) anteroposterior and (b) lateral views of a left internal carotid artery injection, shows successful coil embolization of both the superior and inferior components of the residual aneurysm. After coiling, the superior component has a residual neck, and the inferior component has residual filling of its anterior aspect, from which the fetal posterior communicating artery (arrow) originates
DISCUSSION
The emergence of flow-diverting stents, which allow endovascular reconstruction of the parent vessel, has dramatically improved our ability to treat difficult lesions, including large and fusiform aneurysms.[4567] Since flow-diverting stents are prone to thromboembolic complications, administration of pre- and post-procedural dual antiplatelet therapy is routine. However, hemorrhagic complications may be exacerbated by dual antiplatelet therapy in patients with ruptured aneurysms who undergo flow diversion.[8] In addition, recent studies have suggested that the use of flow diversion in the setting of a fetal PCOM may lead to treatment failure.[9] Due to these two factors, we believed that flow diversion was an unfavorable option for our patient's lesion.
The surgical options for fusiform aneurysms are limited and frequently involve revascularization.[10] In our case, due to the presence of the fetal PCOM, the affected ICA supplied the entire left hemisphere. Therefore, a high-flow bypass to a proximal artery (e.g., MCA M1 segment) would have been necessary to be hemodynamically sufficient. These bypasses are technically demanding and require a period of temporary ischemia which places a large cortical territory at risk of infarction. Another treatment option for some fusiform aneurysms is either surgical or endovascular parent vessel occlusion. However, in our case, we did not believe that collateral flow through the anterior communicating artery would have adequately supplied the at-risk vascular territory without supplementation from a high-flow bypass.
Therefore, we performed a staged multimodal treatment which partially reconstructed the fusiform aneurysm and temporarily secured the rupture site in the first microsurgical stage and then obliterated the remainder of the aneurysm in the second endovascular stage. This combination therapy allowed direct aneurysm intervention without the need for endovascular stenting or microsurgical bypass. While complete clip reconstruction of a fusiform aneurysm functions as external flow diversion, incomplete clip reconstruction can convert a fusiform aneurysm into one or more saccular aneurysms. In our case, the initial fusiform aneurysm was effectively converted into two narrow-necked saccular aneurysms which could be endovascularly coil without the use of adjunctive devices.
CONCLUSIONS
Clip-assisted coiling may be a reasonable alternative to microsurgical bypass and endovascular flow diversion for appropriately selected large fusiform aneurysms presenting with SAH. In many cases, partial clip reconstruction of a ruptured fusiform aneurysm is less technically demanding than a bypass, and it does not expose the distal cortical territory to temporary ischemia. In addition, endovascular occlusion of the residual aneurysm with coiling alone does not necessitate the administration of dual antiplatelet therapy, which is required after endoluminal stent placement.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Giant intracranial aneurysms: Experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-95.
- [Google Scholar]
- Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res. 2014;5:338-46.
- [Google Scholar]
- Tumor necrosis factor-a modulates cerebral aneurysm formation and rupture. Transl Stroke Res. 2014;5:269-77.
- [Google Scholar]
- Endovascular treatment of fusiform intracranial aneurysms. J Neurointerv Surg. 2013;5:110-6.
- [Google Scholar]
- Technology developments in endovascular treatment of intracranial aneurysms. J Neurointerv Surg. 2016;8:135-44.
- [Google Scholar]
- Cavernous carotid aneurysms: A new treatment paradigm in the era of flow diversion. Expert Rev Neurother. 2017;17:155-63.
- [Google Scholar]
- Endovascular treatment of ophthalmic artery aneurysms: Ophthalmic artery patency following flow diversion versus coil embolization. J Neurointerv Surg. 2016;8:919-22.
- [Google Scholar]
- Flow diverter devices in ruptured intracranial aneurysms: A single-center experience. J Neurosurg 2017:1-7. [Epub ahead of print]
- [Google Scholar]
- Treatment failure of fetal posterior communicating artery aneurysms with the pipeline embolization device. J Neurointerv Surg. 2016;8:945-8.
- [Google Scholar]
- Combined microsurgical PICA-PICA bypass and endovascular parent artery occlusion for a ruptured dissecting vertebral artery aneurysm. Neurosurg Focus. 2015;38 VideoSuppl 1:Video3
- [Google Scholar]






