Translate this page into:
Spinal cord compression as initial presentation of metastatic occult follicular thyroid carcinoma
Address for correspondence: Dr. Md Nuruzzaman Khan, Department of Neurosurgery, Sher-e-Bangla Medical College, Barisal, Bangladesh. E-mail: Drkhosru71@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Metastatic tumors are the most common tumors of the spine, accounting for 98% of all spine lesions. But spinal cord compression as the initial presentation of metastatic occult follicular carcinoma without any thyroid enlargement is unusual and relatively rare. This report describes a 35-years-old female patient presenting with paraplegia and urinary incontinence for the last two months. She had no thyroid enlargement; no thyroid related symptoms and her biochemical thyroid profile was normal. Magnetic resonance imaging (MRI) of spine shows a huge mass compressing the spinal cord at D11-D12 involving both the spinal and paraspinal areas. The patient was treated by surgery and radioiodine ablation as the histopathology showed metastatic follicular thyroid carcinoma. This case was reported because of the rarity of the disease. Early diagnosis and initiation of the treatment should promise a good prognosis for a patient with metastatic spinal cord compression.
Keywords
Occult follicular thyroid carcinoma
spinal cord compression
spinal metastasis
Introduction
Metastatic spinal tumors are the most common tumors of the spine, accounting for 98% of all spine lesions. The commonest malignancies that metastasize to the spine include breast (21%), lung (14%), prostate (7.5%), renal (5%), gastrointestinal (5%) and thyroid (2.5%).[1] Among the thyroid malignancies, follicular thyroid carcinoma represents less than 10%. This tumor is usually well encapsulated and often demonstrate vascular invasion and spread via vascular channel.[2] Bone is the second most common site of metastasis resulting from thyroid cancer, after the lung.[3] In contrast, follicular thyroid carcinoma is the most common histological origin of bone metastasis with an incidence ranging from 7% to 28%.[45] Metastasis to the bone, specifically to the vertebral column, may present as bone pain, pathological fracture, or cord compression and is frequently a surgical issue.[6] However, the spinal cord compression, as a complication of thyroid carcinoma, is uncommon. The literature review showed most of the metastatic follicular carcinoma had obvious thyroid swelling and many cases had previous thyroid surgery. But, spinal metastasis of occult follicular carcinoma without any thyroid enlargement or without any thyroid related symptoms is unusual and relatively rare and because of this rarity of the disease, this case was reported. Metastatic thyroid carcinoma should be considered in the differential diagnosis of every patient with new onset spinal cord compression.
Case Report
A 35-years-old female patient presented with the complaints of the pain on the middle part of her back for eight months. Initially, the pain was exacerbated by standing or walking and subsided by rest but later, the patient took some medications to get relief of the pain. About five months ago, she noticed a small swelling on the middle part of the right side of her back but there was no associated pain or discharge or any skin change over the area. The swelling was gradually increasing in size. Then she noticed weakness and heaviness of both legs along with gradual wasting of leg muscles and some extent of difficulties on walking for the last four months. But for the last two months, the patient was totally unable to stand or walk. The patient complained of loss of weight but there was no history of fever. The patient's bladder and bowel habit was normal. The patient had no history of hypertension, diabetes mellitus, bronchial asthma, tuberculosis, jaundice or any neck swelling. The patient had no history of smoking, betel nut chewing or alcohol intake.
On physical examination, she was mildly anemic, normotensive and wasting of calf muscles. There was an ill defined, non tender and firm lump on the right paravertebral region at the level of D11-L1 [Figure 1]. On neurological examination, there was a marked loss of muscle function with grades 1/5 strength in both lower extremity muscles with increased the muscle tone. Ankle and knee reflexes were exaggerated. Planter reflex was extensor and both ankle and patellar clonus present. All modalities of sensations diminished from D11. The X-ray of dorso-lumber region showed bony destruction of transverse process of L1 and pedicles of D12 and L1 [Figure 2]. A magnetic resonance imaging (MRI) scan showed a huge mass involving the right para spinal area and spinal cannel at D11-L1, compressing the spinal cord (D11-D12) and destroyed the posterior arches of the vertebra (D12-L1) [Figure 3a and b].
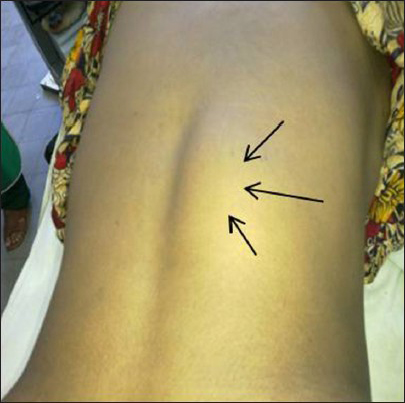
- Arrows indicating right paravertebral swelling
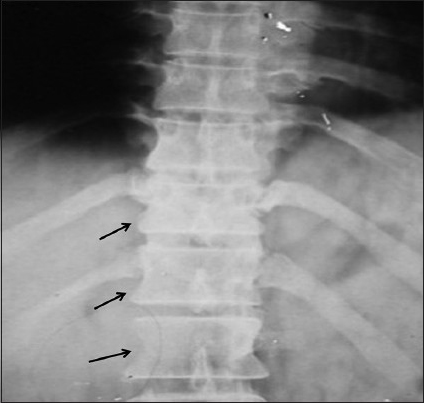
- X-ray of dorsal spine showed (arrow marks) transverse process of L1 (Rt.) and pedicles of D12 and L1 nonvisualized (Destroyed)
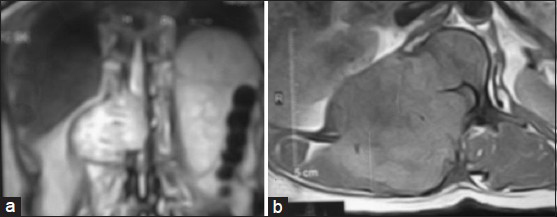
- A magnetic resonance imaging scanT1W images A-coronal and B-axial section showed a huge mass involving the right para spinal area and spinal cannel at D11-L1, compressing the spinal cord (D11-D12) and destroyed the posterior arches of the vertebra (D12-L1)
Decompression of spinal cord by laminectomy of D11- L1 with excision of tumor from both intra-spinal and para-spinal region was done. Tissue was sent for histopathological examination [Figure 4a]. On histopathological examination, the specimen microscopically appears to be thyroid tissue showing follicular adenoma. As it is taken from the vertebra, it is regarded as metastatic follicular carcinoma [Figure 4b and c].
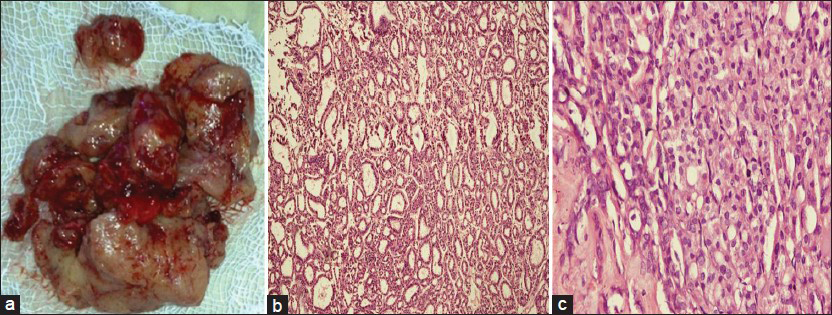
- (a) Excised tumor. (b-c) Microphotograph of histopathological section showed follicular carcinoma of thyroid
Then again the clinical examination of thyroid gland was done which was found not enlarged and biochemical thyroid profile (T3, T4 and TSH) was found normal. But, ultrasonography (USG) of thyroid gland shows multinodular goiter. Fine needle aspiration cytology (FNAC) of thyroid gland suggested in favor of nodular goiter. The patient then underwent a near total thyroidectomy, but macroscopically no tumor was found in the extirpated issue. The histopathological examination of the tissue also revealed in favor of a normal thyroid tissue.
The patient was subsequently treated with an ablation with radioiodine. The patient gradually improved her neurological functions. On the follow-up visit after six months, she was quite normal. The patient had no back pain and can walk normally.
Discussion
Metastatic spine tumors are a common and leading problem throughout the world. Between 5% and 10% of all cancer patients develop spinal metastases during the course of their disease.[7] The commonest malignancies that metastasize to the spine include breast (21%), lung (14%), prostate (7.5%), renal (5%), gastrointestinal (5%) and thyroid (2.5%).[1] Among the thyroid cancer only 5% of patients have metastases beyond the cervical or mediastinal area on initial presentation and a spinal metastasis as the presenting feature of thyroid cancer is unusual.[89] However, spinal cord compression as an initial manifestation of newly diagnosed thyroid carcinoma is a rare event. Available data indicate that only a few sporadic cases of spinal cord compression presenting as the initial manifestation of occult thyroid carcinoma have been reported by Fone-Ching Hsiao, et al.[10] Shaha et al.[11] reported that only 4% patients with thyroid cancer presented initially with a distant metastasis. Of all thyroid cancer subtypes, follicular carcinoma is the most likely to present with distant metastasis[11] or to do so as a late event in a long-standing disease.[12] Fornasier and Horne[13] reviewed a series of autopsy and found, out of 374 specimens from patients with malignancies, 140 of whom had metastatic spread to the vertebral bodies. They identified only one thyroid carcinoma that had metastasized to a vertebral body. Barron et al.[14] reviewed 127 autopsies of patients with spinal cord compression resulting from metastatic neoplasms, and found only three of the tumors were of thyroid origin. Haghpanah et al.[15] reported a case of follicular thyroid carcinoma who presented with paraplegia and urinary incontinence also commented that follicular thyroid carcinoma with metastasis rarely presents with clinical picture of spinal cord compression.
Tumor metastasizing to the spinal column is a common clinical occurrence but asymptomatic thyroid malignancy presenting, as paraplegia is uncommon. The most common presenting symptom of patients with symptomatic spinal metastases is pain, which occurs in 83-95% of patients, and may precede the development of other neurological symptoms by weeks or months.[16] Motor dysfunction is the next most common symptom of patients with metastatic disease of the spine. Weakness in one or more muscle groups is found in 60-85% of patients with metastatic spinal cord compression.[1718] Sensory disturbances including anesthesia, hyperesthesia, and paresthesia usually occur in concert with motor dysfunction and pain in the corresponding dermatomal distributions.[19]
For detecting spinal metastases, bone scan (99mTc-MDP) is more sensitive than plain radiographs. Until MRI became widely available, myelogram and CT scan were the best diagnostic modalities for assessing acute spinal cord compression. MRI is the most sensitive and specific modality for imaging spinal metastases. Fluorine-18 fludeoxyglucose (FDG) positron emission tomography (PET) is a well established method to differentiate malignant from benign lesions in the spine or to demonstrate the viability of previously treated spinal tumor metastasis. In thyroid cancer, PET is useful in patients with metastatic poorly differentiated tumors with high thyroglobulin (Tg) levels and negative 131I whole-body scan results.[2021]
Early diagnosis of metastatic spinal disease is important because functional outcome depends on neurologic condition at the time of presentation. Therapeutic intervention can alleviate pain, preserve or improve neurologic function, achieve mechanical stability, optimize local tumor control, and improve quality of life. Treatment options available for metastatic spine tumors include radiation therapy (RT), surgery, and chemotherapy. The appropriate treatment for an individual patient requires a multidisciplinary approach.
Although some patients underwent only surgical treatment and/or external irradiation for the relief of symptoms, an appropriate and intensive treatment of both the metastatic and primary thyroid tumors is required to achieve long-term survival and a good quality of life for the patients. To prevent further neurological deficits, it is usually advisable to initially and promptly stabilize the spine, especially in the context of potential long-term survival. Traditionally, corticosteroids, local radiation treatment, and surgery to the vertebrae were all thought to be important for most patients with spinal cord compression.[22] Stojadinovic et al.[23] recommended surgery as the preferred method for resectable, locoregional recurrence, followed by radioactive iodine (RAI) therapy for iodide-concentrating thyroid cancer, or external-beam radiation for tumors that lack RAI avidity. Those investigators also found that complete palliative debulking of the localized metastatic lesions of follicular thyroid carcinoma may be associated with an improvement in the patient's survival and quality of life.
Solitary distant metastases of follicular thyroid carcinoma are very rarely amenable to complete resection and thus some local procedures to delay tumor progression and for symptom palliation are used, such as embolization, radio frequency, or cement injection and treatment with bisphosphonates.[24] However, both the locoregional recurrence rate and the mortality rate were reduced to about 25% in patients treated with RAI.[25] A recent review of the literature indicated that although external radiotherapy in association with RAI therapy has an effect on cancer recurrence, pain relief, and the recalcification of osteolytic lesions, external radiotherapy per se cannot improve the survival rate.[26] Instead, complete removal of any tumoral bone tissue in patients less than 45 years of age and a cumulative dose of RAI therapy appeared to improved survival in patients with bone metastases originating from follicular thyroid carcinoma.[27]
The prognosis of occult thyroid carcinoma with distant metastasis also remains a source of contention. Proye et al.[28] demonstrated that follicular carcinoma is usually less life threatening, and that early diagnosis and appropriate treatment for distant metastases can significantly prolong the life span and improve life quality. Shaha et al.[3] also reported that total thyroidectomy followed by RAI therapy and thyroxine suppressive treatment extended long-term survival (10-15 years) in 44% of patients with metastatic follicular thyroid carcinoma. However, Pittas et al.[29] reported that the 10- year survival of patients diagnosed with bone metastasis was only 13%. The patient's refusal of further treatment and the inevitable significant co-morbidities probably have a major adverse effect on survival.
Conclusion
Spinal cord compression is an emergency situation needs prompt diagnosis and treatment. The management of this medical emergency should be a multidisciplinary effort. Prompt management of the primary carcinoma and the metastatic lesion, ongoing maintenance of thyroid suppression, and consideration of the patient's age, response to therapies, and co-morbidities, may extend long-term survival and allow a favorable prognosis. Finally, it is recommended that thyroid carcinoma should be considered in the differential diagnosis of every patient with new onset spinal cord compression.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Bailey and love's Short practice of surgery. (25th ed). United Kingdom: Hodder Arnold; 2008.
- [Google Scholar]
- Occult follicular thyroid carcinoma: An unusualpresentation of multiple lytic bony metastasis in the skull of a 66-year malay man. Ibrahim Med Coli J. 2007;1:25-2.
- [Google Scholar]
- Distant metastasesfrom thyroid and parathyroid cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:243-9.
- [Google Scholar]
- Follicular carcinoma of the thyroidgland: Trends and treatment. Surgery. 1984;96:972-80.
- [Google Scholar]
- Bone metastases from thyroidcarcinoma: A histopathologic study with clinical correlates. Arch Pathol Lab Med. 2000;124:1440-7.
- [Google Scholar]
- Spinal cord compression as a primary manifestation of occult thyroid carcinoma. An Med Interna. 1992;9:334-6.
- [Google Scholar]
- Solitary vertebral column metastasis from occult sclerosing carcinoma of the thyroid gland: Report of a case. Am J Clin Pathol. 1970;53:596-601.
- [Google Scholar]
- Metastatic spinal cord compression as initial presentation of occult follicular thyroid carcinoma. J Med Sci. 2008;28:89-94.
- [Google Scholar]
- Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-6.
- [Google Scholar]
- Distant metastases in differentiated thyroid carcinoma: A multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67:501-8.
- [Google Scholar]
- Experience with metastatic neoplasms involving the spinal cord. Neurology. 1959;9:91-106.
- [Google Scholar]
- Paraplegia as initial presentation of follicular thyroid carcinoma. J Coll Physicians Surg Pak. 2006;16:233-4.
- [Google Scholar]
- Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107:37-43.
- [Google Scholar]
- Epidural spinal cord compression from metastatic tumor: Results with a new treatment protocol. Ann Neurol. 1980;8:361-6.
- [Google Scholar]
- Symptoms and signs in metastatic spinal cord compression: A study of progression from first symptom until diagnosis in 153 patients. Eur J Cancer. 1994;30:396-8.
- [Google Scholar]
- Molecular imaging of potential bone metastasis from differentiated thyroid cancer: A case report. J Med Case Rep. 2011;5:522.
- [Google Scholar]
- Current molecular imaging of spinal tumors in clinical practice. Mol Med. 2011;17:308-16.
- [Google Scholar]
- Metastatic epidural spinal cord compression: Update on management. Semin Oncol. 2006;33:307-11.
- [Google Scholar]
- The role of operations for distantly metastatic well-differentiated thyroid carcinoma. Surgery. 2002;131:636-43.
- [Google Scholar]
- New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol. 2007;8:148-56.
- [Google Scholar]
- Follicular thyroidcarcinoma: Prognostic factors and the role ofradioiodine. Cancer. 2002;95:488-98.
- [Google Scholar]
- Nuclear medicine in treating differentiated thyroid carcinoma. J Med Sci. 2006;26:83-92.
- [Google Scholar]
- Survival and therapeutic modalitiesin patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86:1568-73.
- [Google Scholar]
- Is it still worthwhileto treat bone metastases from differentiated thyroidcarcinoma with radioactive iodine.? World J Surg. 1992;16:640-5.
- [Google Scholar]
- Bone metastases from thyroidcarcinoma: Clinical characteristics and prognostic variablesin one hundred forty-six patients. Thyroid. 2000;10:261-8.
- [Google Scholar]






