Translate this page into:
Short-segment percutaneous fusion versus open posterior fusion with screw in the fractured vertebra for thoracolumbar junction burst vertebral fracture treatment
*Corresponding author: Andrea Perna, Department of Orthopaedics and Traumatology, Fondazione Casa Sollievo della Sofferenza IRCCS, San Giovanni Rotondo, Italy. perna.andrea90@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Perna A, Franchini A, Gorgoglione F, Barletta F, Moretti B, Piazzolla A, et al. Short-segment percutaneous fusion versus open posterior fusion with screw in the fractured vertebra for thoracolumbar junction burst vertebral fracture treatment. J Neurosci Rural Pract. 2024;15:34-41. doi: 10.25259/JNRP_370_2023
Abstract
Objectives:
The treatment options for thoracolumbar junction burst fractures remain a topic of controversy. Short-segment percutaneous fixation (SSPF) and short-segment open fixation including the fractured level (SSOFIFL) are both viable procedures for managing these fractures. At present, there is a lack of evidence in the literature demonstrating the absolute superiority of one treatment over the other. This study aimed to compare these two surgical strategies with a focus on radiological and clinical outcomes.
Materials and Methods:
This retrospective case–control multicenter analysis involved patients with A3 and A4 vertebral fractures at the thoracolumbar junction (T11–L2) who underwent surgical treatment with either SSPF or SSOFIFL in the participating centers. Clinical outcomes were measured using the Oswestry Disability Index and visual analogue scale (VAS) both pre- and postoperatively. Radiological outcomes included kyphotic deformity (KD), anterior vertebral body height (AVBH), segmental kyphosis, and sagittal alignment parameters.
Results:
A total of 156 patients were enrolled in the study, with 81 patients in Group A (SSPF) and 75 patients in Group B (SSOFIFL). Group B demonstrated better correction of KD (Group B: 3.4 ± 2.7° vs. Group A: 8.3 ± 3.2°, P = 0.003), AVBH, and sagittal alignment. A minor loss of correction was observed in Group B with respect to Group A (0.9 ± 1.7° vs 4.3° ± 2.1°, P = 0.043). Blood losses were lower in Group A (78 ± 15 min vs. 118 ± 23 min, P = 0.021) as well as during surgery (121.3 ± 34 mL vs. 210.2 ± 52 mL, P = 0.031), but the post-operative hemoglobin levels were comparable between the two groups.
Conclusion:
SSOFIFL appears to show a major amount of KD correction and prevent loss of correction. This technique should be the preferred choice whenever possible. However, SSPF can be considered a valid alternative for damage control in polytrauma patients and fractures with low KD.
Keywords
Thoracolumbar burst fractures
Minimally invasive systems
Short-segment fixation
Spinal surgery
Percutaneous pedicle screws
INTRODUCTION
Vertebral burst fractures (VBFs) are a fairly common occurrence representing approximately 20% of all vertebral fractures.[1] Over half of VBFs occur at the thoracolumbar junction, which refers to the level between T11 and L2.[2,3] There are two age-related peaks in the incidence of VBFs. The first peak typically occurs in young patients due to high-energy traumas such as car accidents or falls from significant heights. The second peak is more common in elderly patients and is often caused by low-energy traumas.[4]
The optimal treatment for thoracolumbar burst fractures (A3–A4) remains a matter of debate, as per Vaccaro’s AO Spine classification.[5] Conservative treatment could be an option in the absence of neurological injuries or mechanical instability.[6] However, surgical intervention is increasingly becoming one of the preferred treatments. Various surgical techniques are available for treating VBFs.[7]
One surgical method, known as short-segment percutaneous posterior stabilization (SSPF), includes one vertebra above and one below the fractured vertebra. This technique has been shown to reduce surgical duration, costs, and hospitalization length with satisfactory short-term outcomes for patients.[7] Nonetheless, several studies in the literature highlighted several drawbacks of this technique, such as long-term loss of kyphosis correction, increased segmental kyphosis (SK), and persistent pain. Consequently, many authors recommend the use of two screws at the fractured vertebra level to minimize the risk of mechanical failure.[8]
The objective of our study was to compare the short- and long-term radiographic and clinical outcomes of two treatment approaches: SSPF and short-segment open fixation with inclusion of the fractured level (SSOFIFL), in a cohort of patients with VBFs at the thoracolumbar junction.
MATERIALS AND METHODS
Research design
Patients suffering from A3 to A4 thoracolumbar junctions VBFs and surgically treated at the involved centers between January 2017 and December 2021 were retrospectively analyzed in the present study. The STROBE guidelines were followed during the study execution.[9]
Ethical considerations
This study followed national ethical standards and adhered to the principles outlined in the Helsinki Convention. An ad hoc informed consent aimed at collecting clinical data for scientific use was signed by all enrolled patients. The research was conducted retrospectively, utilizing data collected for clinical purposes. The Institutional Review Board of the involved institutions extensively reviewed the study and determined that it did not require formal ethical approval.
Database creation
For all patients accessing the participating centers in the study, the following data were collected: demographic features, clinical and radiographic outcomes, neurological status assessed using the Frankel score,[10] vertebral fracture classification based on AO Spine guidelines,[5] and pain assessment. At all participating centers, patients who underwent surgical treatment were clinically and radiologically evaluated at regular intervals, including 1, 3, 6, 12, and 24 months post-surgery.
Radiological assessment
All enrolled patients underwent spinal computer tomography scan examinations and anteroposterior/lateral (AP/L) views X-rays at the emergency room. AP/L views and X-ray were also obtained during all follow-up visits. Retrospectively, the following parameters were calculated based on the X-ray images:
Kyphotic deformity (KD): This is defined as the angle formed by the intersection of a plane passing through the upper endplate and a plane passing through the lower endplate of the fractured vertebra.
Anterior vertebral body height (ABVH): This measurement is expressed in millimeters.
SK: SK is defined as the angle formed by the intersection of a plane passing through the upper endplate of the vertebra above and a plane passing through the lower endplate of the vertebra above the fractured ones.
Sagittal index (SI): SI is calculated by measuring KD at the fractured vertebra and adjusting it to the baseline sagittal normal contour (SNC) of the normal spine. The baseline SNC was estimated as an angle of 5° in the thoracic segment, 0° at the thoracolumbar junction, and −10° in the lumbar segment.[11] SI is obtained by subtracting the SNC from KD. A simplified illustration of the examined radiographic parameters is provided in the Figure 1

- Simplification of radiographic parameters measured. AVBH: Anterior vertebral body height, SK: Segmental kyphosis, SI: Sagittal index, SNC: Sagittal normal contour. KD: Kyphotic deformity
The measurements mentioned above were performed by three experienced spinal surgeons (F.L.G., F.B., and A.P.).
Surgical data collection
From the databases of the participating institutions, the following surgical data were extracted for each patient included in the study: Operation duration, estimated blood loss, pre- and post-operative hemoglobin (Hb) values, type of construct used, and length of hospital stay.
Clinical evaluation and complications
Clinical evaluation was conducted using the Oswestry Disability Index (ODI) and visual analog scale (VAS) for back pain, which were recorded during the first access and all follow-up visits. Any complications occurring during the perioperative period and throughout the follow-up period were also documented.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (I) fractured level at thoracolumbar junction; (II) short-segment stabilization (one vertebra above and one below the fractured vertebra); (III) no neurological deficit; and (IV) surgery performed at least 72 h after the trauma.
The exclusion criteria were excluded from the study patients with (I) vertebral infective disease; (II) primary or secondary vertebral malignancy; (III) presence of rheumatic conditions; (IV) lower extremity fractures or severe head trauma resulting in prolonged bed rest; (V) patients with a confirmed diagnosis of osteoporosis (T-score ≤−2.5 on bone densitometry); (VI) history of vertebral surgical operation; (VII) concomitant presence of other spinal fractures; (VIII) extension of the fracture to one or both pedicles; and (IX) use of polymethyl methacrylate augmentation.
Based on the type of surgery received, the patients included in the study were divided into two groups. Group A comprised patients treated with short-segment percutaneous posterior fixation (SSPF), while Group B consisted of patients treated with short-segment open fixation including the fractured level (SSOFIFL).
Surgical technique
We considered an SI value >15° on pre-operative radiographs as the threshold for surgical treatment. Initially, SK correction at the fractured vertebra level was achieved by placing the patient in hyperextension on the operating table. A fluoroscopic C-arm device was utilized for all procedures.
In Group A (SSPF), a fluoroscopic C-arm device was used to obtain an AP image and identify the radiographic landmarks. Small skin incisions (approximately 2 cm) were made and projected on vertebral pedicles. With fluoroscopic guidance, four guide wires were placed in the pedicles of the vertebra above and below the fractured one. Four cannulated pedicle screws were then inserted over the guide wires, with fluoroscopic verification in the AP/L views. Additional kyphosis correction was achieved by lordotic rods and applying tulip compression through the dedicated cannulas. Two minimally invasive systems were used for all patients in this group: Precept (NuVasive, San Diego, CA, US) and CD Horizon Voyager (Medtronic Sofamor Danek USA, Inc.). Polyaxial screws were implanted in these patients. Antibiotic prophylaxis with 2 g of cefazolin was administered to all patients in this group 30 min before the surgical incision. An exemplary representation of Group A patients is provided in the Figure 2.

- An exemplary case of a male 59-year-old patient belonging to Group A: (a) Sagittal view of thoracolumbar spine computer tomography scan with L1 A3 fracture. (b) Pre-operative lateral view spinal X-ray and an angular Kyphosis of 16°, SI = 16. (c) Post-operative lateral view spine X-ray images showing 7° kyphotic deformity improvement. KD: Kyphotic deformity, AVBH: Anterior vertebral body height.
In Group B (SSOFIFL), after determining the surgical level with fluoroscopic guidance in the lateral view, an epispinous skin incision extending to one vertebra below and one above was made. The paravertebral lodges were then exposed to visualize the anatomical landmarks (facet joints, isthmus, and transverse process). Pedicle screws were inserted in the vertebra above and below the fractured one using a free-hand technique. Subsequently, two shorter screws (35 or 40 mm) were placed in the fractured vertebra using the same technique. Further, kyphosis correction was achieved through ligamentotaxis during rod insertion, rod contouring, and compression maneuvers. Before wound closure, sacrifice of the zygapophyseal joints and decortication of the laminae were performed to promote posterior fusion. Two open systems were used for all patients in this group: CD Horizon Solera (Medtronic Sofamor Danek USA, Inc) and Expedium (DePuy Synthes). Monoaxial screws were implanted in these patients. Antibiotic prophylaxis with 2 g of ceftriaxone and 400 mg of teicoplanin was administered to all patients in this group 60 min before the surgical incision. [Figure 3] provides an exemplary representation of group B patients.
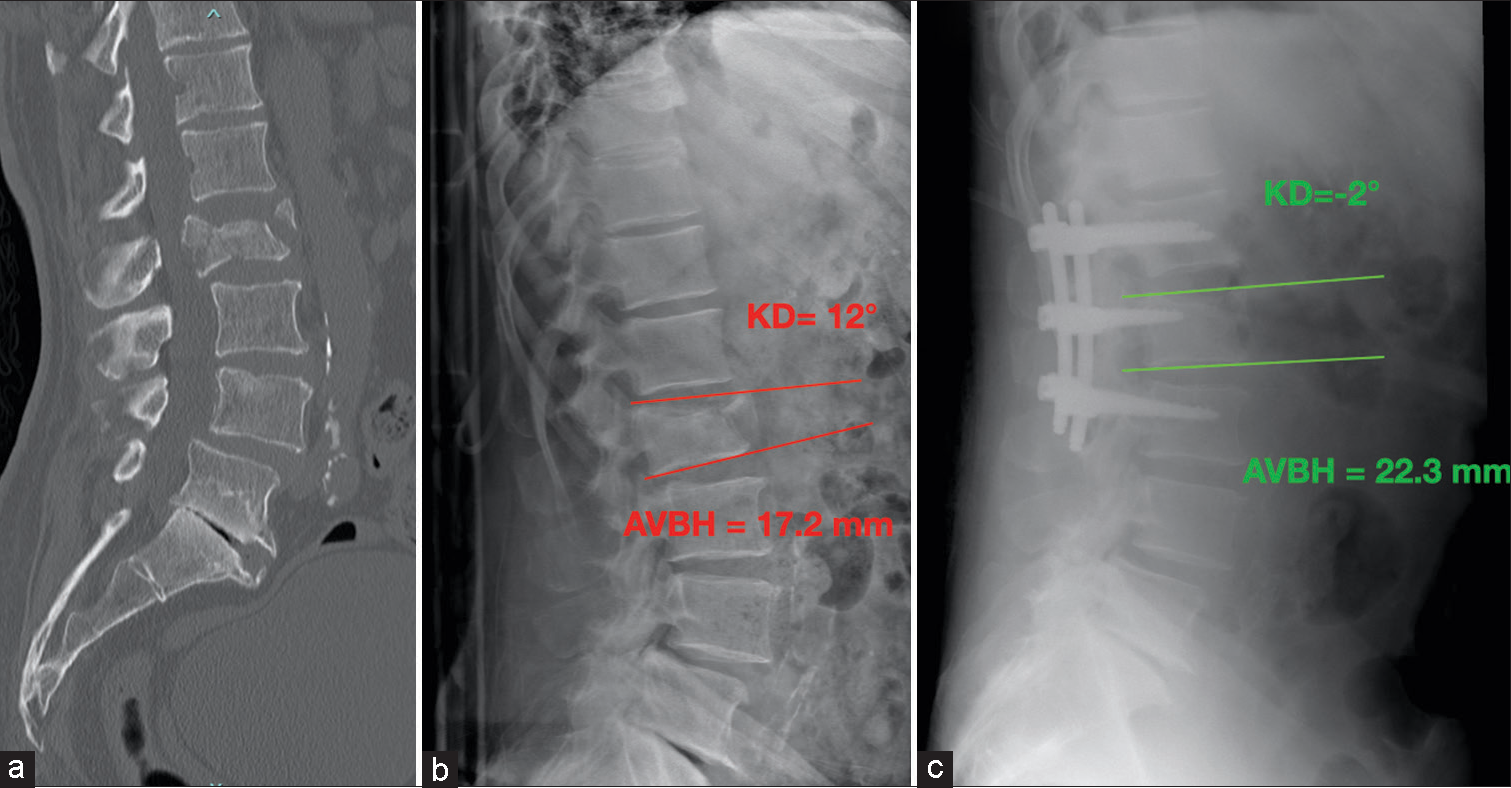
- An exemplary case of a male 57-year-old patient belonging to Group B: (a) Sagittal view of thoracolumbar spine computer tomography scan with L2 A4 fracture. (b) Pre-operative lateral view spinal X-ray and an angular kyphosis of 14°, SI = 22. (c) Post-operative lateral view spine X-ray images showing 13° kyphotic deformity improvement. KD: Kyphotic deformity, AVBH: Anterior vertebral body height
Post-operative care
All patients removed bladder catheters 2 days after surgery. Mobilization in a sitting and standing position took place on the 2nd post-operative day with the help of dedicated physiotherapists. All patients wore a three-point rigid brace for 3 months postoperatively. The surgical wounds were dressed every 3 days until the sutures were removed.
Outcomes
The amount of fractured vertebral kyphosis correction after surgery and the loss of correction after 24 months measured by KD represent the primary outcomes. The clinical and functional outcomes during follow-up visit, intraoperative blood loss, duration of surgery, surgery failure, and implant breakage represent the secondary outcomes.
Statistical analysis
The independent ordinal variables were analyzed using the Mann–Whitney U-test, while the dependent ordinal variables were analyzed employing Wilcoxon Signed-Rank Test. Fleiss’ Kappa statistic was used to determine the inter-rater reliability (IRR) between the three senior spinal surgeon observers on the radiographic measurements. The normality of the sample was analyzed by conducting the Kolmogorov–Smirnov statistic. Considering that a non-normal distribution was obtained, the variance analysis by the Analysis of Variance test could not be performed. A significance level of P < 0.05 was established. Quantitative variables were described using means and standard deviations, whereas qualitative variables were presented as numbers and percentages. All values were reported with only one decimal digit, rounded up. The statistical analysis was conducted using the dedicated SPSS software (SPSS Inc., Chicago, IL).
RESULTS
Seven hundred and twelve records were retrospectively examined, but only 213 fully met the inclusion and exclusion criteria. Fifty-seven patients did not have a complete documentation or were lost to follow-up; thus, 156 patients were enrolled in the study. Of these, based on the treatment performed, 81 patients were assigned to Group A, while 75 were to Group B. The demographic data of the patients are shown in Table 1. No significant differences were found between the two groups, excluding the traumatic mechanism.
| Demographics | Group A (%) | Group B | P-value |
|---|---|---|---|
| No. of patients | 81 | 75 | |
| Age | 55.4 (±15.4) | 56.2 (±16.2) | 0.832 |
| Sex | M: 51; F: 30 | M: 53; F: 22 | |
| BMI | 26.7±2.1 | 27.1±1.8 | 0.741 |
| Diabetes | 6 (7.4) | 8 (10.7) | 0.654 |
| Smokers | 31 (38.3) | 32 (42.7) | 0.729 |
| Other comorbidities | 34 (42) | 33 (44) | 0.824 |
| Type of fracture (AO classification) | |||
| A3 | 54 (66.7) | 43 (57.3) | 0.624 |
| A4 | 27 (33.3) | 32 (42.7) | 0.612 |
| Fractured level | |||
| D11 | 7 (8.6) | 5 (6.8) | 0.749 |
| D12 | 34 (42) | 29 (38.6) | 0.831 |
| L1 | 28 (34.6) | 26 (34.6) | 0.757 |
| L2 | 12 (14.8) | 15 (20) | 0.816 |
| Traumatic mechanism | |||
| Traffic accident | 53 (65.4) | 31 (41.3) | 0.023 |
| High falling injury | 23 (28.4) | 37 (49.3) | 0.034 |
| Other causes | 5 (6.2) | 7 (9.4) | 0.872 |
BMI: Body mass index
The IRR was calculated using the Fleiss’ kappa statistical analysis (0.771, 95% CI: 0.652–0.897). No statistically significant differences were observed between the two groups in the pre-operative values of KD, AVBH, SK, and SI.
As reported in Table 2, the patients of Group B present a better correction of the KD in the post-operative period compared to the patients of Group A, and this data are statistically significant (Group B: 3.4 ± 2.7° vs. Group A: 8.3 ± 3.2°, P = 0.003). This correction is maintained over time as can be seen from the 24-month follow-up data. Similar observations are possible also for the correction of the AVBH and for the SI. The mean loss of correction was about 4.3° (±2.1°) in Group A and about 0.9° (±1.7°) in Group B, and this difference is statistically significant (P = 0.043).
| Radiographic parameters | Group A | Group B | P-value |
|---|---|---|---|
| No. of patients | 81 | 75 | |
| Pre-operative KD | 17.1 (±4.3)° | 18.3 (±5.1)° | 0.236 |
| Post-operative KD | 8.3 (±3.2)° | 3.4 (±2.7)° | 0.003 |
| 24 m FU KD | 12.6 (±2.7)° | 4.3 (±2.1)° | 0.012 |
| Pre-operative AVBH | 14.2 (±7.1) mm | 13.9 (±6.5) mm | 0.362 |
| Post-operative AVBH | 19.3 (±3.6) mm | 23.1 (±2.1) mm | 0.023 |
| 24 m FU AVBH | 18.4 (±2.2) mm | 22.7 (±2.4) mm | 0.041 |
| Pre-operative SK | 11.7 (±5.6)° | 12.1 (±3.9)° | 0.471 |
| Post-operative SK | 8.1 (±4.4)° | 7.3 (±4.7)° | 0.382 |
| 24 m FU SK | 8.7 (±4.8)° | 7.7 (±4.1)° | 0.421 |
| Pre-operative SI | 21.3 (±1.3)° | 22.1 (±1.6)° | 0.328 |
| Post-operative SI | 11.3 (±1.7)° | 6.1 (±0.7)° | 0.013 |
| 24 m FU SI | 13.1 (±1.3)° | 6.9 (±0.8)° | 0.032 |
AVBH: Anterior vertebral body height, FU: Follow-up, KD: Kyphotic deformity, SK: Segmental kyphosis, SI: Sagittal index
No intraoperative complications occurred in the two groups. Comparing the data obtained, the duration of the surgical intervention and the estimated intraoperative blood losses is lower in Group A and the data are statistically significant (respectively 78 ± 15 min versus 118 ± 23 min, P = 0.021 and 121.3 ± 34 mL versus 210.2 ± 52 mL P = 0.031). However, the Hb value 2 days after surgery between the two groups is comparable. No patient required blood transfusions. Surgical data are shown in Table 3.
| Surgical parameters | Group A | Group B | P-value |
|---|---|---|---|
| No. of patients | 81 | 75 | |
| Operative time | 78 (±15) min | 118 (±23) min | 0.021 |
| Estimated | |||
| blood loss | 121.3 (±34) mL | 210.2 (±52) mL | 0.031 |
| Pre-operative | |||
| Hb value | 13.2 (±3.3) g/dL | 13.5 (±4.1) g/dL | 0.372 |
| Post-operative | |||
| Hb value | 11.5 (±4.1) g/dL | 11.9 (±3.8) g/dL | 0.412 |
| Type of instrumentation | |||
| Expedium | - | 27 (36%) | |
| CD Solera | - | 48 (64%) | |
| Precept | 61 (75.3%) | - | |
| CD Voyager | 20 (24.7%) | - | |
| Los (days) | 3.4 (±2.1) | 4.5 (±1.8)° | 0.183 |
Hb: Hemoglobin; Los: Length of stay
No statistically significant variation was found between the pre-operative and immediately post-operative VAS and ODI values between the two groups. On the other hand, Group A showed better VAS values after 1 month of follow-up (3.2 ± 1.9 vs. 7.3 ± 2.1 P = 0.024); however, Group B showed clearly better VAS values after 24 months of follow-up (1.9 ± 1.4 vs. 4.5 ± 1.8; P = 0.029). Group B also showed better ODI values than Group A after 12 and 24 months of follow-up and these differences are statistically significant. Mechanical complications such as screw loosening and instrumentation breakage were much more frequent in Group A than in Group B (10% vs. 1.3%) and this difference is statistically significant. Furthermore, about 11% of patients in Group A had chronic low back pain at the last follow-up compared to 5.3% in Group B. The clinical outcomes and complications data are shown in Table 4.
| Clinical outcomes | Group A | Group B | P-value |
|---|---|---|---|
| Number of patients | 81 | 75 | |
| Pre-operative VAS | 9.1 (+/3.2) | 9.4 (±4.1) | 0.875 |
| Post-operative VAS | 8.9 (±4.2) | 9.3 (±3.7) | 0.761 |
| 1 month FU VAS | 3.2 (±1.9) | 7.3 (±2.1) | 0.024 |
| 6 months FU VAS | 3.7 (±1.2) | 4.2 (±1.9) | 0.652 |
| 12 months FU VAS | 4.6 (±1.5) | 2.3 (±0.9) | 0.045 |
| 24 months FU VAS | 4.5 (±1.8) | 1.9 (±1.4) | 0.029 |
| Pre-operative ODI | 82.7 (±14.2) | 84.1 (±16.3) | >0.05 |
| Post-operative ODI | 72.8 (±18.3) | 77.6 (±19.9) | >0.05 |
| 1 month FU ODI | 54.2 (±12.6) | 66.7 (±15.1) | >0.05 |
| 6 months FU ODI | 32.4 (±11.2) | 34.1 (±10.3) | >0.05 |
| 12 months FU ODI | 29.3 (±9.3) | 18.1 (±8.2) | 0.039 |
| 24 months FU ODI | 27.3 (±10.1) | 16.7 (±9.1) | 0.007 |
| Complications | |||
| Screws loosening | 5 (6.2%) | 1 (1.3%) | 0.021 |
| Implant failure | 3 (3.7%) | - | 0.001 |
| Wound infection | 1 (1.2%) | 1 (1.3%) | 0.233 |
| Chronic pain | 11 (13.6%) | 4 (5.3%) | 0.024 |
FU: Follow-up, ODI: Oswestry disability index, VAS: Visual analog scale
DISCUSSION
The management of thoracolumbar fractures presents ongoing challenges and controversies, with limited evidence and consensus regarding the optimal surgical technique.[4,7] In general, surgical intervention is widely accepted for thoracolumbar fractures with neurological deficits. If there is no neurological deficit or no fracture instability was observed, surgery is often not recommended.[12] The literature suggests that VBFs surgical treatment should be considered when a loss of anterior and middle column height exceeding 50% or when the local Cobb angle of the fractured vertebra is >15–20° took place.[12]
Numerous studies compared open and percutaneous surgery for thoracolumbar fractures.[13-19] Most of these reports indicate that there are no significant differences in clinical outcomes between open and percutaneous surgery for the treatment of unstable VBFs. Minimally invasive surgery is often preferred due to several advantages, including shorter operative time, reduced intraoperative and post-operative bleeding, decreased need for blood transfusions, shorter hospitalization time, lower infection rate, less muscle damage, and faster functional recovery.[13,14]
However, it is worth noting that the percutaneous transpedicular screw system may have limitations in fracture reduction and the restoration of vertebral height.[20] In cases where VBF involves a vertebral height loss of more than 50%, open surgery should be considered, as minimally invasive surgery may not achieve adequate reduction or improve post-traumatic kyphosis.[16]
Nevertheless, it is crucial to consider the overall health condition of the patient when selecting the appropriate treatment approach. Therefore, in patients with poor general health, such as those with spinal metastases from cancer, open treatment may not always be recommended. In such cases, percutaneous treatment is preferable for palliative surgery, as supported by a recent meta-analysis.[21]
During the immediate post-operative period, no significant differences were observed in the clinical course of patients in both groups of our series. Evaluation of the VAS and ODI scales did not reveal any statistically significant differences. However, at the 1-month follow-up after surgery, patients in Group A showed better clinical conditions. Interestingly, at the 12- and 24-month follow-up, patients in Group B demonstrated significantly better outcomes in terms of VAS and ODI values. A recent study by Perna et al. highlighted an interesting correlation between early loss of kyphosis correction and clinical outcomes in patients with thoracolumbar junction VBFs treated with Single-Screw Posterior Fixation (SSPF).[4] They observed that patients who experienced a kyphosis loss of correction exceeding 2° in the 1st month after surgery exhibited a clear worsening of functional outcomes and back pain. In our study, the mean loss of correction was approximately 4.3° in Group A and 0.9° in Group B and this difference was statistically significant. Furthermore, Group B patients demonstrated superior correction of key radiographic parameters, including KD, sacral inclination (SI), and AVBH in the post-operative period, with statistically significant data. Moreover, this correction was maintained over time. The loss of correction observed in Group A with multiaxial screws could be explained by the possibility of slight movement persisting between the screw tulip and the rod, even after inner locking. Therefore, whenever possible, monoaxial screws should be preferred for this type of surgery. Other studies, such as Cappuccio’s ones, have also shown a worsening of SK in patients treated with polyaxial screws during radiographic follow-up at 12 months.[22] Chung et al. have emphasized the difference in results obtained when placing percutaneous monoaxial screws compared to open procedures, highlighting that improper placement of monoaxial screws may cause difficulties in rod passage and inner displacement.[23] This could explain the clinical results observed in Group B, as all the analyzed radiographic parameters (AVBH, KD, SK, and SI) at the 24-month follow-up were better. In contrast, 11 patients in Group A developed chronic pain at the surgical site. In addition, a higher number of mechanical failures were observed in Group A. Specifically, among patients in Group B, only one case of screw loosening was reported. In contrast, Group A patients experienced eight cases of mechanical complications, including five implant failures and three instances of screw loosening. Seven of these patients required revision surgery. Other studies have also reported complications following percutaneous stabilization, such as a high rate of mechanical complications with screw or rod disconnection.[22,24] For instance, in Cappuccio’s study, four mechanical complications were reported, including screw head disconnection from the rod in two patients on the 1st post-operative day and pedicle screw pullout occurring 15 and 20 days postoperatively. All these cases required surgical revision.[22]
In our study, two groups were treated with two different types of screws. Group A with polyaxial screws while Group B with monoaxial screws. Therefore, the loss of correction of Group A with multiaxial screws could be explained by the possibility that a slight movement persists between the screw tulip and the rod, even after locking the inner. This could explain the better clinical result of Group B; in fact, all the radiographic parameters analyzed AVBH, KD, SK, SI, and a FU at 24 months are better while 11 patients in Group A developed chronic pain at the site of surgery.
Regarding estimated intraoperative blood loss, our study showed significantly lower blood losses in Group A, although post-operative Hb levels were comparable between the two groups. This suggests that blood losses with the percutaneous approach are often occult. In fact, most patients undergoing percutaneous treatment developed a back soft-tissue hematoma. Other studies, although with smaller patient populations, have reported complication rates ranging from 8% to 14%.[22-25] Despite the reduction in skin incision and soft-tissue injury associated with the percutaneous approach, moderate complications such as hematoma, wound infections, and delayed wound healing have been observed and required surgical revision.[24] The occurrence of bleeding may be related to the length of the surgical incision. In this regard, the difference in length between a single incision and four smaller incisions for screw insertion, as well as two additional incisions for rod insertion, is only few millimeters.
Although the percutaneous approach reduces skin incision and soft-tissue dissection, in the immediate post-operative period, no differences were found in the clinical course of the patients in both groups
Limitations
The present study has certain limitations. First, its retrospective design poses a limitation. Second, the study employed stringent exclusion criteria, resulting in a highly selected patient cohort. Finally, the involvement of three different surgical teams may introduce bias. Future prospective studies are required to validate the findings observed in this study. Moreover, the two examined techniques are biomechanically distinct. In fact, a mechanical superiority was anticipated for the SSOFIFL technique compared to the SSPF technique. An appropriate investigation is required to assess how the femoral obliquity angle and the T1 pelvic tilt angle may impact the overall alignment and influence clinical outcomes in cases of lumbar fractures.[26]
The objectives of a future study will be to compare the SSOFIFL technique with the short-segment percutaneous fixation including the fractured level technique.
CONCLUSION
Both techniques examined in the study could be used for the surgical treatment of VBFs at the thoracolumbar junction. In our patient cohort, SSOFIFL allows a better and more durable correction of KD over time than SSPF; this may explain the better long-term clinical outcomes. In our opinion, SSOFIFL should preferably be used whenever possible. The use of SSPF should be reserved for polytrauma patients for damage control and in fractures with low KD.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817-31.
- [CrossRef] [PubMed] [Google Scholar]
- The incidence and distribution of burst fractures. Emerg Radiol. 2006;12:124-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acute thoracolumbar burst fractures the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984;189:142-9.
- [CrossRef] [Google Scholar]
- Early loss of angular kyphosis correction in patients with thoracolumbar vertebral burst (A3-A4) fractures who underwent percutaneous pedicle screws fixation. J Orthop. 2021;24:77-81.
- [CrossRef] [PubMed] [Google Scholar]
- AOSpine thoracolumbar spine injury classification system: Fracture description, neurological status, and key modifiers. Spine (Phila Pa). 2013;38:2028-37.
- [CrossRef] [PubMed] [Google Scholar]
- A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184-201.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of unstable thoracolumbar junction fractures: Short-segment pedicle fixation with inclusion of the fracture level versus long-segment instrumentation. Acta Neurochir (Wien). 2016;158:1883-9.
- [CrossRef] [PubMed] [Google Scholar]
- The use of screw at the fracture level in the treatment of thoracolumbar burst fractures. J Spinal Disord Tech. 2009;22:417-21.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and prognosis of traumatic spinal cord injury. Global Spine J. 2011;1:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Sagittal index in management of thoracolumbar burst fractures. Spine (Phila Pa 1976). 1990;15:958-65.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of RTS versus percutaneous conventional pedicle screw fixation on Type A thoracolumbar fractures: A retrospective cohort study. Eur Spine J. 2020;29:2484-90.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive percutaneous fixation in the treatment of thoracic and lumbar spine fractures. Eur Spine J. 2009;18(Suppl 1):71-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of multifidus muscle atrophy and trunk extension muscle strength: Percutaneous versus open pedicle screw fixation. Spine (Phila Pa 1976). 2005;30:123-9.
- [CrossRef] [Google Scholar]
- Percutaneous short fixation vs conservative treatment: Comparative analysis of clinical and radiological outcome for A.3 burst fractures of thoraco-lumbar junction and lumbar spine. Eur Spine J. 2014;6:671-6.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous versus open pedicle screw fixation for treatment of thoracolumbar fractures: Systematic review and meta-analysis of comparative studies. Clin Neurol Neurosurg. 2015;135:85-92.
- [CrossRef] [PubMed] [Google Scholar]
- Open versus minimally invasive fixation techniques for thoracolumbar trauma: A Meta-analysis. Global Spine J. 2016;6:186-94.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous versus traditional and paraspinal posterior open approaches for treatment of thoracolumbar fractures without neurologic deficit: A meta-analysis. Eur Spine J. 2017;26:1418-31.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive spine surgery in the treatment of thoracolumbar and lumbar spine trauma. Neurosurg Focus. 2014;37:E11.
- [CrossRef] [PubMed] [Google Scholar]
- A novel, percutaneous, self-expanding, forceful reduction screw system for the treatment of thoracolumbar fracture with severe vertebral height loss. J Orthop Surg Res. 2018;13:174.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior percutaneous pedicle screws fixation versus open surgical instrumented fusion for thoracolumbar spinal metastases palliative management: A systematic review and meta-analysis. Front Oncol. 2022;12:884928.
- [CrossRef] [PubMed] [Google Scholar]
- Complications in minimally invasive percutaneous fixation of thoracic and lumbar spine fractures. Orthopedics. 2013;36:e729-34.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive reduction of thoracolumbar burst fracture using monoaxial percutaneous pedicle screws: Surgical technique and report of radiological outcome. J Orthop Surg (Hong Kong). 2020;28:2309499019888977.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of complications and perioperative data after open or percutaneous dorsal instrumentation following traumatic spinal fracture of the thoracic and lumbar spine: A retrospective cohort study including 491 patients. Eur Spine J. 2017;26:1535-40.
- [CrossRef] [PubMed] [Google Scholar]
- The feasibility of long-segment fluoroscopy-guided percutaneous thoracic spine pedicle screw fixation, and the outcome at two-year follow-up. Malays Orthop J. 2019;13:39-44.
- [CrossRef] [PubMed] [Google Scholar]
- The role of femoral obliquity angle and T1 pelvic angle in predicting quality of life after spinal surgery in adult spinal deformities. BMC Musculoskelet Disord. 2021;22(Suppl 2):999.
- [CrossRef] [PubMed] [Google Scholar]






