Translate this page into:
Sensory profile and its impact on quality of life in patients with painful diabetic polyneuropathy
Address for correspondence: Dr. Kongkiat Kulkantrakorn, Neurology Division, Department of Internal Medicine, Faculty of Medicine, Thammasat University Rangsit Campus, Pathumthani - 12120, Thailand. E-mail: kongkiat1@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Painful diabetic polyneuropathy (PDN) is common and causes significant disability. The sensory profile in each patient is different and affects quality of life.
Aim:
To describe the demographic, details of sensory profile and its impact on quality of life in patients with PDN.
Settings and Design:
A cross-sectional survey in patients with PDN who were treated in a University Hospital.
Materials and Methods:
They were interviewed with standard questionnaires, which included neuropathic pain scale (NPS), a short-form McGill Pain Questionnaire (SF-MPQ) and a short form-36 quality of life survey (SF-36).
Statistical Analysis Used:
Descriptive statistics were used in demographic data. Student's t test was used to analyze continuous data. Multiple comparisons for proportions and correlations were made using Fisher Exact test and Pearson's coefficient of correlation, respectively.
Results:
Thirty three patients were included in this study. In NPS, sharp pain was the most common symptom and itching was the least common. Almost all patients had more than one type of pain. The mean VAS was 53 mm. In SFMPQ, the sensory score, affective score and the present pain score fell in the moderate range. In SF-36, physical functioning was the most affected and social function was the least affected.
Conclusions:
PDN significantly affects patients’ quality of life, especially physical function and role limitation due to a physical problem. Almost all patients have many types of pain and sharp pain is the most common.
Keywords
Pain
polyneuropathy
quality of life
sensory profile
type 2 diabetes
Introduction
Neuropathy is one of the most common complications in both type I and type II diabetes mellitus. It can present in various forms, as both focal or symmetric types. The most common form is a chronic, symmetrical, length-dependent axonal sensorimotor polyneuropathy. Some patients are asymptomatic, but many patients have sensory symptoms, either negative or positive ones. These symptoms may fluctuate over time. Some of them also have pain associated with neuropathy, also known as painful diabetic neuropathy (PDN), which is strongly associated with other diabetic complications.
On the other hand, painful diabetic neuropathy is the most common cause of neuropathic pain.[1] The diagnosis of PDN is a clinical one, which relies on the patient's description of pain.[2] The symptoms are distal, symmetrical, often associated with nocturnal exacerbations, and commonly described as prickling, deep aching, sharp pain, like an electric shock, and burning with hyperalgesia and frequently allodynia upon examination.[2]
These symptoms were always categorized using rating scales and standard pain questionnaires to assess the frequency and severity of painful symptoms, and treatment responses. Moreover, each type of pain is believed to be caused by different pathophysiological mechanisms, therefore, each neuropathic pain medication might have a different effect on sensory symptoms.[2]
Having PDN also has a significant negative effect on quality of life, especially in physical terms, and a significantly worse trajectory of quality of life outcomes over time and long-term increased total costs.[34]
Subjects
Patients of either sex who had type II diabetes for at least one year, and were older than 20 years old and had had PDN for at least one month were recruited from the internal medicine and neurology clinic at a University Hospital in the northern suburban area of Bangkok, Thailand. Each subject had to meet all of the following PDN diagnostic criteria: 1) the patient's medical history and absence of other causes of neuropathy after additional investigations, 2) presence of signs and symptoms of typical clinical features of distal symmetric sensory polyneuropathy, such as numbness, burning pain, sharp pain, loss of pain or touch sensation, etc., and 3) score more than four in neuropathic pain diagnostic questionnaires (Thai DN4).[5] They were recruited between February and December, 2010.
Clinical methods
This is a descriptive study of patients with PDN with regards to their demographic information, sensory profile and quality of life. It was approved by the ethical committee of the Faculty of Medicine, Thammasat University.
From February 2010 to December 2010, 33 patients were approached to participate in this study. After obtaining informed consent from each patient, prior to the inclusion in the study, demographic information was collected from the patients who were screened positive for PDN. They were asked to complete questionnaires, which included a Neuropathic Pain Scale (NPS)[6] to describe the pain quality and its severity, a Short-Form McGill Pain Questionnaire (SF-MPQ)[7] where the higher score indicates worse pain, and the Thai version of the Short Form-36 Quality of Life Questionnaire (SF-36)[8] Pain severity was assessed by visual analog scale (VAS).
Statistical analysis
All analyses were conducted using the computer program, SPSS for Windows (version 13.0; SPSS, Inc., Chicago, IL, USA). Data were presented as proportions or means (± S.D.). Descriptive statistics were used in demographic data. Student's t test was used to analyze continuous data, for example VAS and quality of life. Multiple comparisons for proportions and correlations were done using Fisher Exact test and Pearson's coefficient of correlation, respectively. The level of significance was taken as a P < 0.05.
Results
Thirty three patients were included in this study. Their details were broken down by gender and are summarized below in Table 1. There was no significant difference in demographic information, except height.
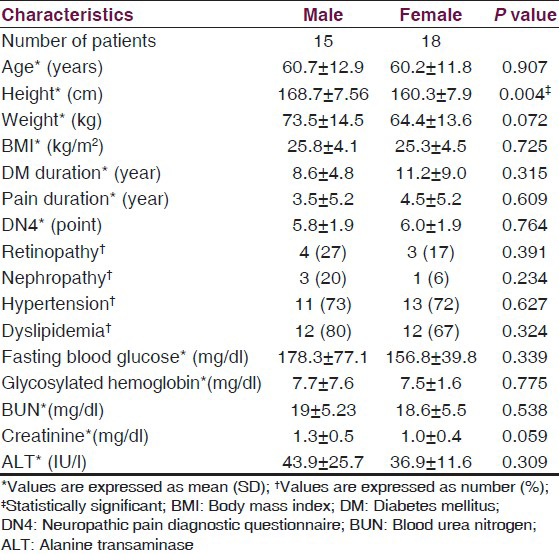
Regarding pain characteristics, NPS, SF-MPQ and SF-36 data were reported in Tables 2–4. From NPS, the most common type of pain was sharp pain and the least common one was itching. The unpleasantness score was 6.9. This was high. The SF-MPQ score showed an average score across all components. The average VAS was 53 mm. Out of the ten types of pain listed in the NPS questionnaires, 14 of 33 (42.2%) had four types of pain or more, 11 of 33 (33.3%) had three types, 7 of 33 (21.1%) had two types and only one out of 33 (3.03%) of patients had one type. Multiple comparisons and association analysis between pain intensity, pain type and baseline characteristics revealed that high pain intensity and presence of any types of pain were significantly associated with the female subjects (P = 0.03) and in subjects with creatinine level less than 1.2 mg/dl (P < 0.01).

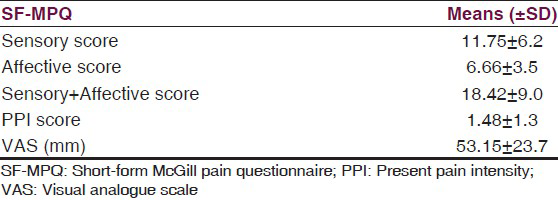
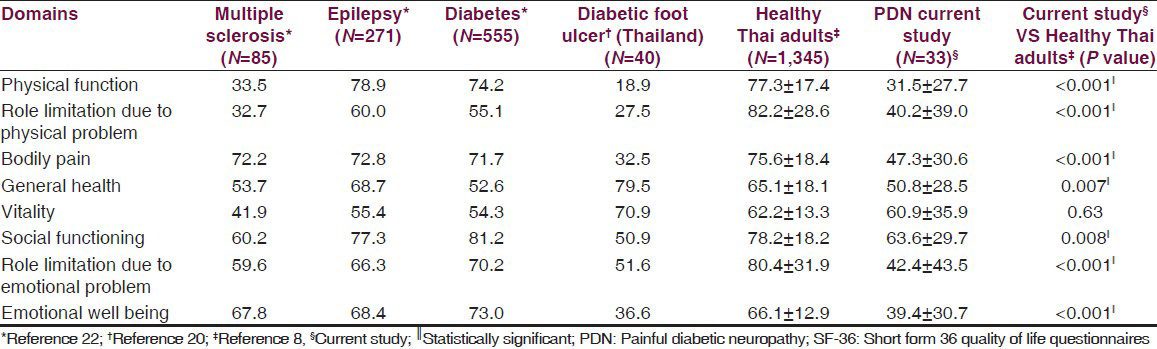
Regarding SF-36, the physical function was most affected and the social function was least affected. The SF-36 data from each disease and healthy subjects were compared in Table 4.
Discussion
DPN is still a clinical diagnosis, despite the advance in neurophysiologic studies. Incorporating standard pain questionnaires in clinical evaluation will aid in early diagnosis and better management in these patients.[9] In this study, we used a DN4 questionnaire, which has been validated as a reliable screening tool for neuropathic pain in diabetic patients with the cut-off point score of 4.[10] The questionnaires can be used to screen and differentiate between neuropathic and non-neuropathic pain.[11]
The sensory profile in our DPN patients showed that sharp pain is the most common and the least common is itching. This NPS and SF-MPQ data is in line with previous reports.[1213] Almost all patients had more than one type of pain which adds more complexity to any clinical evaluation. This may imply that the mechanism of pain is most likely from small nerve fibers, rather than from large fiber dysfunction. Previous clinical and electrophysiological studies also confirmed that neuropathic pain in diabetic polyneuropathy is not associated with the degree of involvement of large diameter sensory fiber or diabetes severity.[14]
Interestingly, when looking at the sharp pain, the duration of diabetes was not associated with painful symptoms. This is due to the history of small fiber neuropathy which can occur in pre-diabetes stage.[15] Although the pain of PDN may resolve completely over time in some patients, in those in whom painful neuropathic symptoms had persisted over five years, no significant improvement in pain intensity was observed.[16] In our patients, the mean duration of pain was four years. This indicated the persistency of pain and it was probably less likely to resolve spontaneously. Female subjects were predictive of pain,[9] as in this present study. However, creatinine levels lower than 1.2 mg/dl was also associated with a higher risk of PDN, which has never been reported before and needed to be confirmed in the future with larger dataset.
The presence of DPN significantly affected patients’ quality of life, especially physical function. Moreover, it was associated with significantly worse trajectory of quality of life outcomes over time and long-term increased total costs, when compared to patients with non-painful diabetic polyneuropathy. The presence and severity of neuropathic pain were associated with greater impairments in a number of important health-related quality of life (HRQoL) domains.[31718] This data was in line with previous reports in diabetic patients whether they had PDN or not.[192021] When comparing with the normal healthy Thai population with regards to the SF-36 subcategories[8] in Table 4, DPN had a detrimental effect in all subcategories, except vitality. Moreover, PDN patients had poorer physical function than other chronic neurological illnesses or than the general diabetic population [Table 4].[22] Their quality of life (QOL) was similar to diabetic foot ulcer patients which indicated severe disability.[20]
A recent American Academy of Neurology evidence-based review has used VAS as a primary measure and physical function and QOL, e.g., using SF-36 as guidelines for an efficient assessment to formulate the recommendation for pharmacological treatment of painful diabetic polyneuropathy (PDN).[23] However, in a clinical trial situation, quantitative sensory testing (QST) is still necessary for a more objective measurement of outcomes, as well as HRQoL.[2425]
There were some strengths and limitations of this study which should be mentioned. This study was based on an outpatient clinic population which reflected a real-life practice. However, it was a single center study with a small number of patients. A quantitative sensory test was not performed to confirm the neuropathy. However, it might not have been necessary in this real life practice survey.
Despite the improvement in treatment modalities for chronic pain in recent years, patients with PDN continue to be inadequately treated. The different profiles of pain quality and spatial characteristics suggest that the assessment of patterns of pain symptoms might contribute to the identification of distinct pathophysiological mechanisms, subgroups of patients and the development of mechanism-based treatment approaches.[26] This will eventually improve the outcome and quality of life in these patients.
Source of Support: Faculty of Medicine, Thammasat University.
Conflict of Interest: None declared.
References
- EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. In: Eur J Neurol. Vol 17. 2010. p. :1113-e88.
- [Google Scholar]
- Neuropathic pain: Diagnosis, pathophysiological mechanisms and treatment. In: Lancet Neurol. Vol 9. 2010. p. :807-19.
- [Google Scholar]
- A longitudinal assessment of painful diabetic peripheral neuropathy on health status, productivity, and health care utilization and cost. Pain Med. 2011;12:118-26.
- [Google Scholar]
- Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-93.
- [Google Scholar]
- Cross-cultural adaptation to the Thai language of the neuropathic pain diagnostic questionnaire (DN4) J Med Assoc Thai. 2007;90:1860-5.
- [Google Scholar]
- Development and preliminary validation of a pain measure specific to neuropathic pain: The Neuropathic Pain Scale. Neurology 1997:332-8.
- [Google Scholar]
- Thai SF-36 health survey: Tests of data quality, scaling assumptions, reliablity and validity in healthy men and women. Health Qual Life Outcomes. 2008;6:52.8.
- [Google Scholar]
- Importance of pain evaluation for more accurate diagnosis of painful diabetic polyneuropathy. Medicina (Kaunas). 2010;46:735-42.
- [Google Scholar]
- Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med. 2012;29:578-85.
- [Google Scholar]
- The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518-22.
- [Google Scholar]
- A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146:34-40.
- [Google Scholar]
- Distal symmetric polyneuropathy in diabetes: Differences between patients with and without neuropathic pain. Exp Clin Endocrinol Diabetes. 2012;120:45-50.
- [Google Scholar]
- The natural history of chronic painful peripheral neuropathy in a community diabetes population. Diabet Med. 2006;23:1021-4.
- [Google Scholar]
- The impact of neuropathic pain on health-related quality of life: Review and implications. Neurology. 2007;68:1178-82.
- [Google Scholar]
- Efficacy of oxcarbazepine in the treatment of painful diabetic neuropathy. Clin J Pain. 2004;20:174-8.
- [Google Scholar]
- A multidisciplinary diabetic foot protocol at Chiang Mai University Hospital: Cost and quality of life. Int J Low Extrem Wounds. 2009;8:153-6.
- [Google Scholar]
- A randomized clinical trial of the effectiveness of photon stimulation on pain, sensation, and quality of life in patients with diabetic peripheral neuropathy. Pain Symptom Manage. 2010;39:88-99.
- [Google Scholar]
- A comparison of health-related quality of life in patients with epilepsy, diabetes and multiple sclerosis. Epilepsy Res. 1996;25:113-8.
- [Google Scholar]
- American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758-65.
- [Google Scholar]
- Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain. 2009;25:641-7.
- [Google Scholar]
- Symptom profiles differ in patients with neuropathic versus non-neuropathic pain. J Pain. 2007;8:118-26.
- [Google Scholar]
- Fibromyalgia and neuropathic pain-differences and similarities: A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurol. 2011;11:55.
- [Google Scholar]






