Translate this page into:
Ruptured Brain Arteriovenous Malformations: Surgical Timing and Outcomes—A Retrospective Study of 25 Cases
Alessandro Di Bartolomeo, MD Department of Neurological Sciences, Neurosurgery, “La Sapienza” University of Rome 00161 Rome Italy diba.ale@gmail.com
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Background One important problem in treatment of ruptured brain arteriovenous malformations (bAVMs) is surgical timing. The aim of the study was to understand which parameters affect surgical timing and outcomes the most.
Materials and Methods Between January 2010 and December 2018, 25 patients underwent surgery for a ruptured bAVM at our institute. Intracerebral hemorrhage (ICH) score was used to evaluate hemorrhage severity, while Spetzler-Martin scale for AVM architecture. We divided patients in two groups: “early surgery” and “delayed surgery.” The modified Rankin Scale (mRS) evaluated the outcomes.
Results Eleven patients were in the “early surgery” group: age 38 ± 18 years, Glasgow Coma Scale (GCS) 7.64 ± 2.86, ICH score 2.82 ± 0.71, hematoma volume 45.55 ± 23.21 mL. Infratentorial origin of hemorrhage was found in 27.3% cases; AVM grades were I to II in 82%, III in 9%, and IV in 9% cases. Outcome at 3 months was favorable in 36.4% cases and in 54.5% after 1 year. Fourteen patients were in the “delayed surgery” group: age 41 ± 16 years, GCS 13.21 ± 2.39, ICH score 1.14 ± 0.81, hematoma volume 29.89 ± 21.33 mL. Infratentorial origin of hemorrhage was found in 14.2% cases; AVM grades were I to II in 50% and III in 50%. Outcome at 3 months was favorable in 78.6% cases and in 92.8% after 1 year.
Conclusions The early outcome is influenced more by the ICH score, while the delayed outcome by Spetzler-Martin grading. These results suggest that it is better to perform surgery after a rest period, away from the hemorrhage when possible. Moreover, this study suggests how in young patient with a high ICH score and a low AVM grade, early surgery seems to be a valid and feasible therapeutic strategy.
Keywords
cerebral hemorrhage
ruptured brain arteriovenous malformation
treatment
Introduction
Arteriovenous malformations (AVMs) constitute a specific subgroup of lesions that can affect each structure of the brain, with a prevalence between 70 and 93% at the level of supratentorial structures.1 They consist of an anomalous tangle of modified, high-flow vessels characterized by the shunting of arterial blood into draining veins in the absence of a physiological capillary bed. The average reported detection rate for this condition is 1.34 per 100,000 person-years.2 Although AVMs can be silent for many years, in more than 50% of the cases clinical onset occurs with hemorrhage.2 About 2% of all intracranial hemorrhage is due to rupture of a brain arteriovenous malformation (bAVM). AVM-related hemorrhage results in varying degrees in morbidity with a risk of permanent neurological deficits of 30 to 50% and mortality of 10 to 30%.3 Current literature data show that AVM rupture is associated with a risk of rebleeding ranging from 6 to 39% per year following the initial hemorrhage.4 5 6
While definitive treatment is fairly standardized,7 surgical timing in case of ruptured bAVM is less standardized. International literature2 8 9 10 recommends, in general, a variable “rest period” (1–6 weeks) between the hemorrhage and the conclusive treatment.
The aim of the study was to clarify, through a retrospective analysis of a multioperator series, (1) which parameters should be considered in the choice of the best surgical timing in case of ruptured bAVMs and (2) how these parameters can influence outcomes.
Materials and Methods
Between January 2010 and June 2018, 32 patients affected by bAVMs underwent surgery in the Neurosurgery Department of Sapienza University of Rome. Of these, 25 met the inclusion criteria (radiological evidence of intracranial hemorrhage) and were therefore analyzed.
For each patient we evaluated: age, sex, pre-existing medical comorbidities, onset symptoms and signs, discharge symptoms and signs, angioarchitectural characteristics of the lesion (including size, location, and venous drainage), bleeding severity, treatment modalities, surgical timing, and outcomes at 3 and 12 months. AVMs were investigated using computed tomography angiography, magnetic resonance imaging angiography, and/or traditional angiography. The Spetzler-Martin AVM grading system was applied to the classification of the lesions.11 The presence or absence of aneurysms on feeding arteries was also taken into account. Risk stratification, in relation to bleeding severity, was evaluated through the use of intracerebral hemorrhage (ICH) score that takes into consideration: (1) Glasgow Coma Scale (GCS), (2) hematoma volume (mL), (3) infratentorial origin (yes/no), (3) intraventricular hemorrhage (IVH) (yes/no), and (4) age (years). Regarding surgical timing, we divided patients into two groups: (1) “early surgery” (timing ≤ 48 hours, 11 patients) and (2) “delayed surgery” (timing> 48 hours, 14 patients). In each case, histological examination confirmed the diagnosis of AVM.
ICH score and time of surgery for each patient are showed in Table 1.
|
Age |
No. of patients |
|---|---|
|
Abbreviations: AVM, arteriovenous malformation; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale. |
|
|
<40 y ≥40 y |
11 14 |
|
Sex |
|
|
Male Female |
8 17 |
|
GCS |
|
|
GCS ≥ 14 GCS 9–13 GCS ≤ 8 |
11 6 8 |
|
Hemorrhage location |
|
|
Typical location Atypical location |
5 20 |
|
Presence of intraventricular hemorrhage |
|
|
Yes No |
11 14 |
|
ICH volume |
|
|
<30 mL >30 mL |
12 13 |
|
Supra/infratentorial hemorrhage |
|
|
Supratentorial Infratentorial |
20 5 |
|
Spetzler-Martin grade |
|
|
I or II (Spetzler-Ponce class A) III (Spetzler-Ponce class B) IV (Spetzler-Ponce class C) |
16 8 1 |
|
Presence of AVM-related aneurysms |
|
|
Yes No |
7 18 |
|
Surgical timing |
|
|
Timing ≤ 48 h Timing ≥ 48 h |
11 14 |
|
Treatment modalities |
|
|
Grade I–II Surgery (microsurgical resection) Only evacuation of hematoma |
15 1 |
|
Grade III Surgery (microsurgical resection) Multimodal (endovascular and surgical) treatment |
1 7 |
|
Grade IV Only evacuation of hematoma |
1 |
|
Outcomes |
|
|
Mortality |
3 |
|
mRS at 3 mo favorable (mRS ≤ 2) unfavorable (mRS ≥ 3) |
17 8 |
|
mRS at 1 y favorable (mRS ≤ 2) unfavorable (mRS ≥ 3) |
19 6 |
We employed the modified Rankin Scale (mRS) to assess outcomes in terms of degree of disability/dependence following the acute event.12
Univariate and multivariate analysis of the collected data were performed using Excel, MATLAB and Stata. For each parameter, we calculated mean and standard deviation. We also estimated correlation, covariance, and relation coefficient with the ordinary least squares (OLS) linear regression model.
Results
Results of the cohort analysis are described in Tables 1 2 3 4, and in Figs. 1 2.
|
Patients |
Surgical timing |
Age (y) |
GCS |
Hematoma volume (mL) |
Infratentorial hemorrhage |
Intraventricular hemorrhage |
ICH score |
|---|---|---|---|---|---|---|---|
|
Abbreviations: GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage. |
|||||||
|
Pt. 1 |
1 h |
24 |
8 |
41 |
No |
No |
2 |
|
Pt. 2 |
9 h |
9 |
8 |
12 |
Yes |
Yes |
4 |
|
Pt. 3 |
3 h |
22 |
12 |
62.5 |
No |
Yes |
3 |
|
Pt. 4 |
3 h |
23 |
4 |
55.3 |
No |
No |
3 |
|
Pt. 5 |
21 h |
40 |
12 |
89.4 |
No |
No |
2 |
|
Pt. 6 |
24 h |
80 |
10 |
42 |
No |
No |
3 |
|
Pt. 7 |
4 h |
43 |
3 |
10 |
Yes |
Yes |
4 |
|
Pt. 8 |
2 h |
17 |
7 |
57.5 |
No |
No |
2 |
|
Pt. 9 |
15 h |
40 |
5 |
13.86 |
No |
Yes |
2 |
|
Pt. 10 |
45 h |
77 |
6 |
75 |
No |
Yes |
3 |
|
Pt. 11 |
48 h |
44 |
9 |
42.5 |
Yes |
No |
3 |
|
Pt. 12 |
90 d |
43 |
14 |
32 |
No |
No |
1 |
|
Pt. 13 |
6 d |
22 |
6 |
16.4 |
No |
Yes |
2 |
|
Pt. 14 |
74 d |
40 |
13 |
45 |
Yes |
No |
2 |
|
Pt. 15 |
8 d |
23 |
11 |
24 |
No |
No |
1 |
|
Pt. 16 |
7 d |
29 |
15 |
24 |
No |
Yes |
1 |
|
Pt. 17 |
13 d |
43 |
11 |
60 |
No |
No |
2 |
|
Pt. 18 |
15 d |
69 |
14 |
13.5 |
No |
No |
0 |
|
Pt. 19 |
6 d |
57 |
14 |
46.62 |
No |
Yes |
2 |
|
Pt. 20 |
21 d |
32 |
15 |
13.4 |
No |
No |
0 |
|
Pt. 21 |
44 d |
62 |
14 |
24 |
No |
Yes |
1 |
|
Pt 22 |
9 d |
51 |
15 |
12.5 |
No |
No |
0 |
|
Pt. 23 |
8 d |
64 |
13 |
3 |
Yes |
Yes |
2 |
|
Pt. 24 |
53 d |
23 |
15 |
6.08 |
No |
No |
0 |
|
Pt. 25 |
6 d |
17 |
15 |
146.5 |
No |
Yes |
2 |
|
Patients |
AVM diameter (cm) |
Location (eloquent or not eloquent cortex) |
Venous drainage |
Spetzler-Martin grade |
mRS at 3 mo |
mRS at 1 y |
|---|---|---|---|---|---|---|
|
Abbreviations: AVM, arteriovenous malformation; D, deep; mRS, modified Rankin Scale; S, superficial. |
||||||
|
Pt. 1 |
1.5 |
Eloquent |
S |
II |
2 |
1 |
|
Pt. 2 |
1.7 |
Eloquent |
S |
II |
3 |
1 |
|
Pt.3 |
1.5 |
Eloquent |
S |
II |
3 |
1 |
|
Pt. 4 |
2.5 |
Not eloquent |
S |
I |
2 |
1 |
|
Pt. 5 |
2.6 |
Eloquent |
S |
II |
4 |
3 |
|
Pt. 6 |
1.5 |
Eloquent |
S |
II |
6 |
6 |
|
Pt. 7 |
1.7 |
Eloquent |
S |
II |
6 |
6 |
|
Pt. 8 |
2.5 |
Eloquent |
S and D |
II |
1 |
0 |
|
Pt. 9 |
2 |
Eloquent |
S |
III |
2 |
1 |
|
Pt. 10 |
3.5 |
Eloquent |
D |
IV |
5 |
5 |
|
Pt 11 |
1 |
Not eloquent |
D |
II |
6 |
6 |
|
Pt. 12 |
2.8 |
Not eloquent |
S |
I |
2 |
1 |
|
Pt. 13 |
2.5 |
Eloquent |
S |
III |
3 |
2 |
|
Pt. 14 |
3.2 |
Eloquent |
S |
III |
4 |
3 |
|
Pt. 15 |
5 |
Eloquent |
S |
III |
1 |
1 |
|
Pt 16 |
1.3 |
Not eloquent |
S |
I |
0 |
0 |
|
Pt. 17 |
2 |
Eloquent |
S |
II |
3 |
2 |
|
Pt. 18 |
0.8 |
Eloquent |
S |
II |
2 |
1 |
|
Pt. 19 |
2.5 |
Not eloquent |
S |
I |
2 |
1 |
|
Pt. 20 |
3.5 |
Eloquent |
S |
III |
2 |
1 |
|
Pt. 21 |
8 |
Not eloquent |
S |
III |
1 |
1 |
|
Pt. 22 |
4 |
Eloquent |
S |
III |
2 |
2 |
|
Pt. 23 |
3.5 |
Not eloquent |
S and D |
III |
1 |
1 |
|
Pt. 24 |
1 |
Not eloquent |
S |
I |
1 |
1 |
|
Pt. 25 |
2.4 |
Eloquent |
S |
II |
2 |
1 |
|
Early postoperative complications |
Early surgery (%) |
Delayed surgery (%) |
|---|---|---|
|
Neurological deficits |
36 (4 pts) |
21 (3 pts) |
|
Post-surgical hemorrhage |
18 (2 pts) |
0 (0 pts) |
|
Post-surgical ischemia |
9 (1 pt) |
0 (0 pts) |
|
Infection |
9 (1 pt) |
0 (0 pts) |
|
Seizures |
18 (2 pts) |
7 (1 pt) |
|
Pulmonary embolism |
9 (1 pt) |
0 (0 pts) |
|
Death |
27 (3 pts) |
0 (0 pts) |
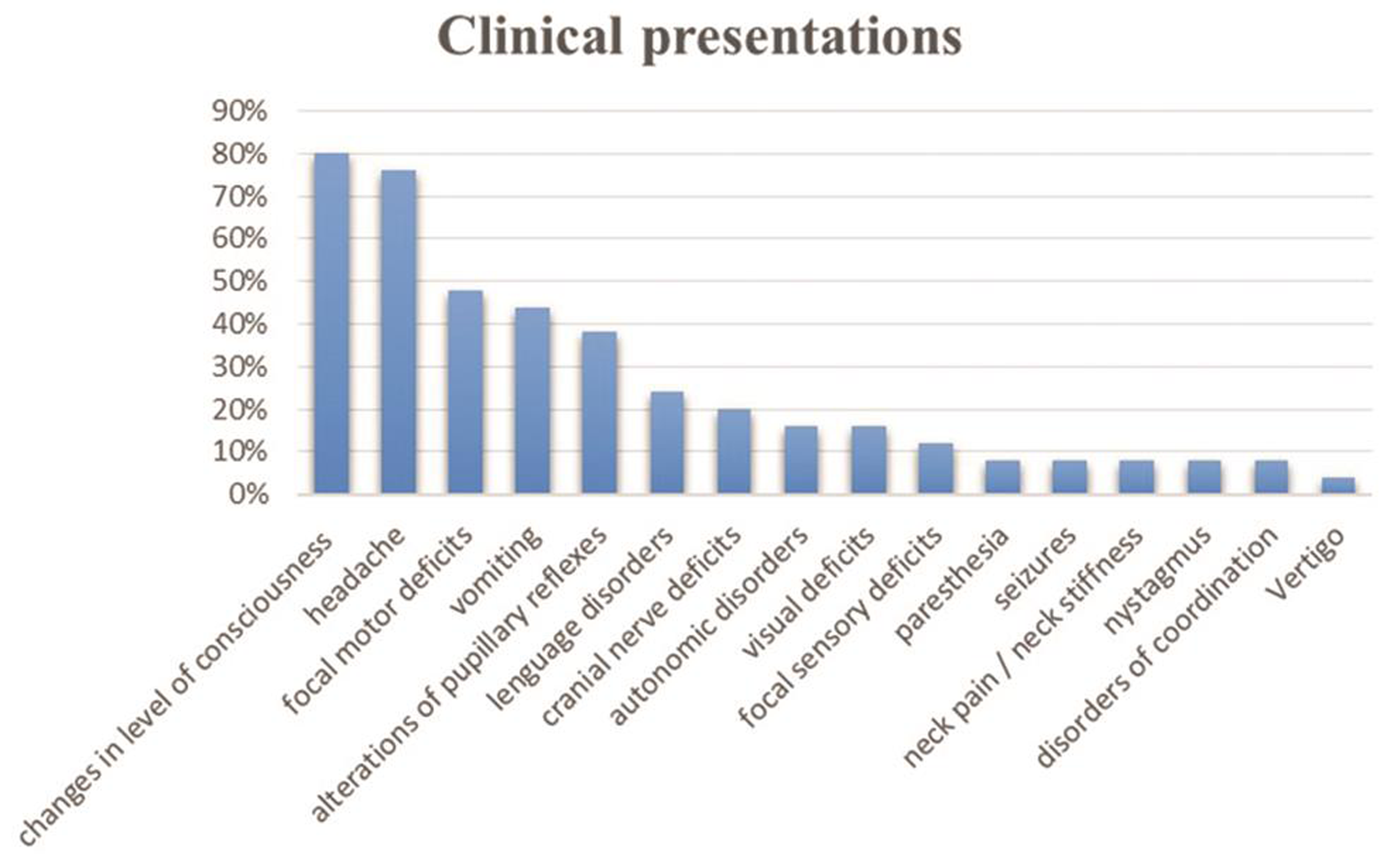
-
Fig. 1 Clinical presentations.
Fig. 1 Clinical presentations.
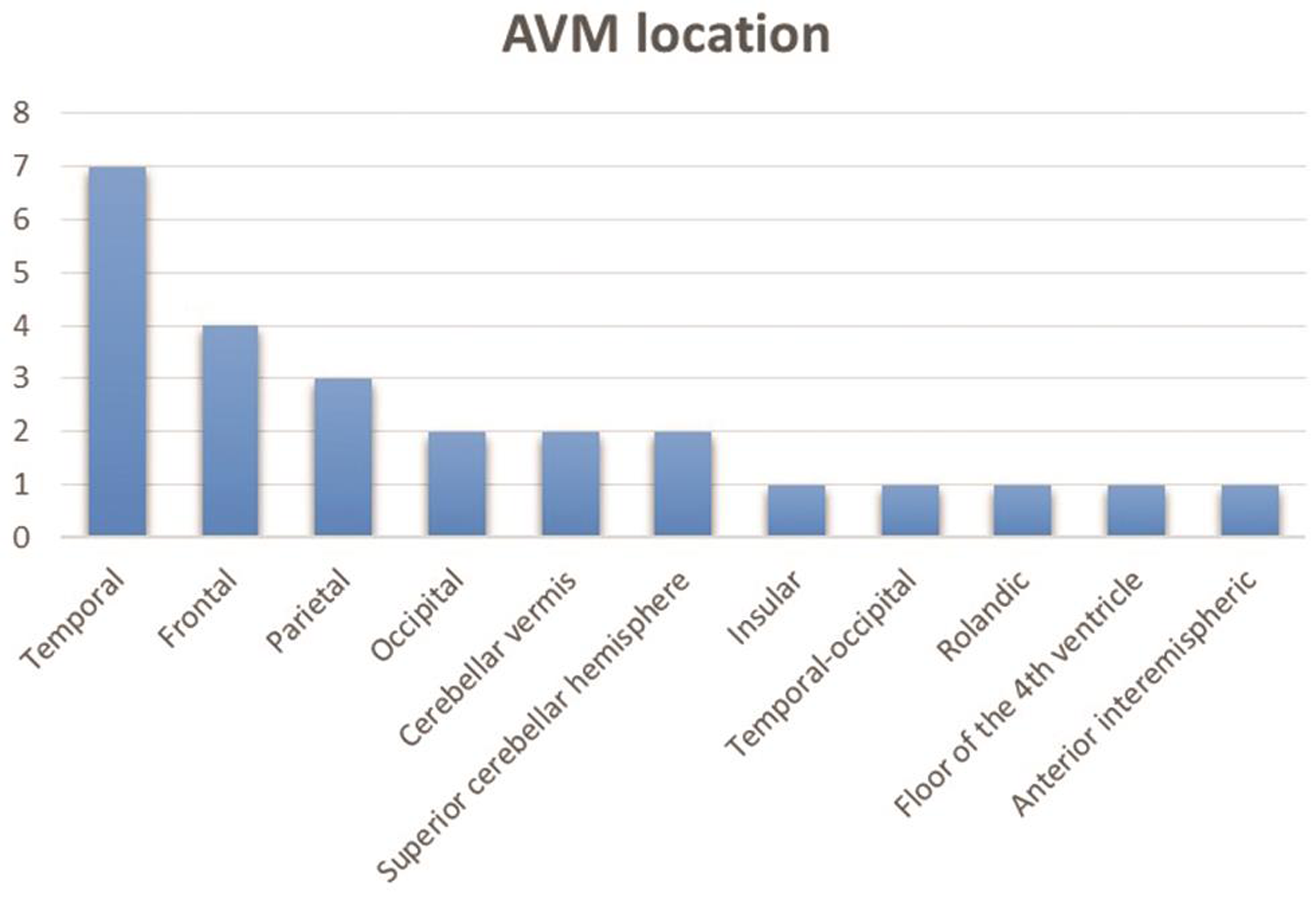
-
Fig. 2 Arteriovenous malformation (AVM) location.
Fig. 2 Arteriovenous malformation (AVM) location.
Trying to respond to our objectives, we focused our attention on the parameters analyzed below.
ICH Score, Surgical Timing, and Outcome
The mean ICH score for the “early surgery” subgroup of patients was 2.82 ± 0.71, while it was 1.14 ± 0.81 for the “delayed surgery” one. Patients with a favorable outcome (mRS≤2) at 3 months and 1 year had a mean ICH score, respectively, of 1.26 ± 0.72 and of 1.57 ± 0.92, while those with a poor outcome (mRS>2) at 3 months and 1 year had a mean ICH score respectively of 2.8 ± 0.71 and of 2.8 ± 0.63. We found a positive statistical correlation (ρ) between bleeding severity and outcome, given the calculated values: 0.63 at 3 months and 0.50 at 1 year. Covariance (σ) (ICH-score, mRS) at 3 months and 1 year was respectively 1.24 and 1.07, both positive (the two values positively covary together).
Besides, through a linear regression model (OLS), we calculated the regression coefficient of the variable ICH score against mRS, obtaining these results: 0.91 at 3 months and 0.79 at 1 year. So, an increase in 1 unit of the ICH score implies an increase in the expected mRS score of 0.91 at 3 months and of 0.79 at 1 year (Figs. 3 4).
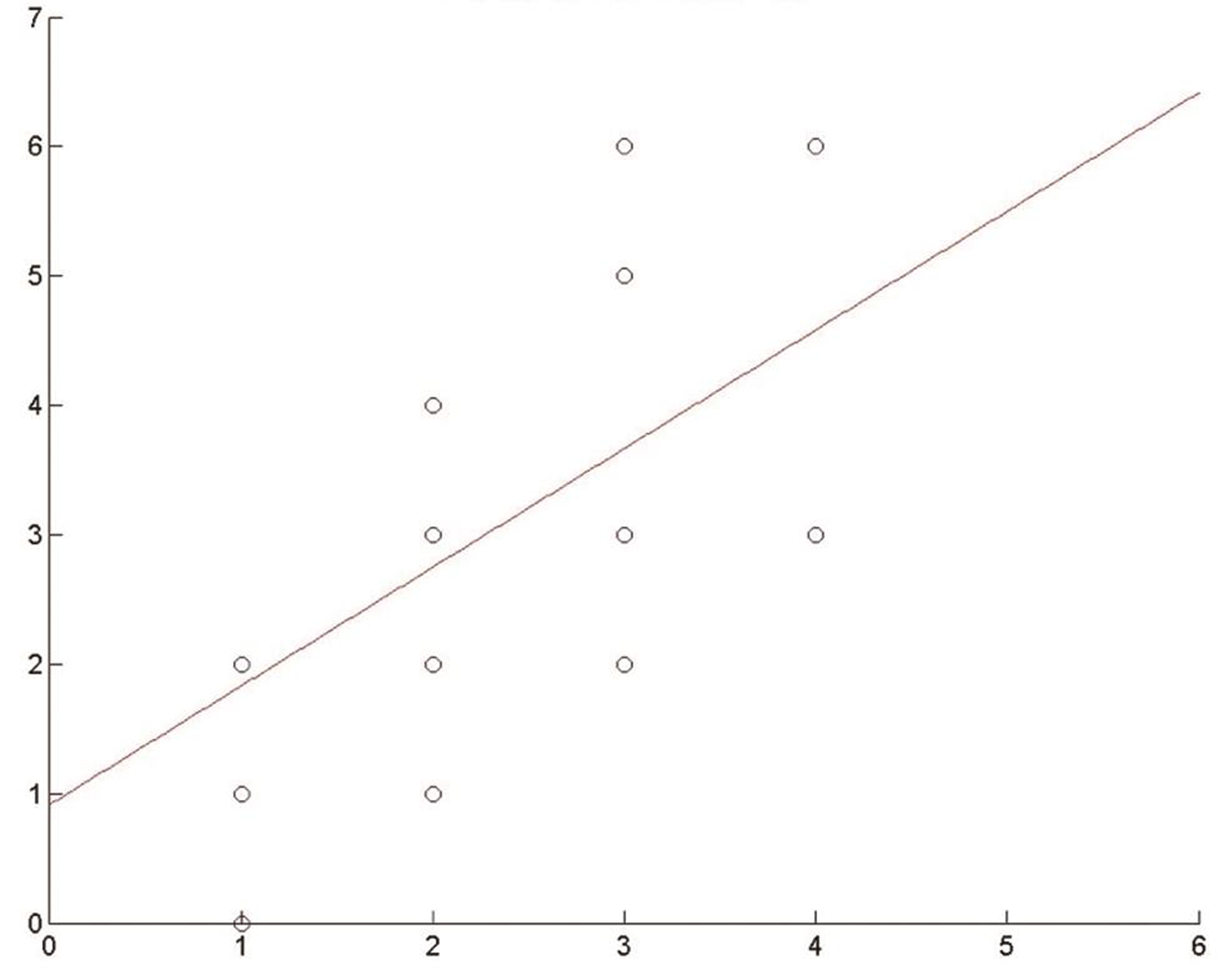
-
Fig. 3 Effect of intracerebral hemorrhage (ICH) score on modified Rankin Scale (mRS). Regression coefficient at 3 months.
Fig. 3 Effect of intracerebral hemorrhage (ICH) score on modified Rankin Scale (mRS). Regression coefficient at 3 months.
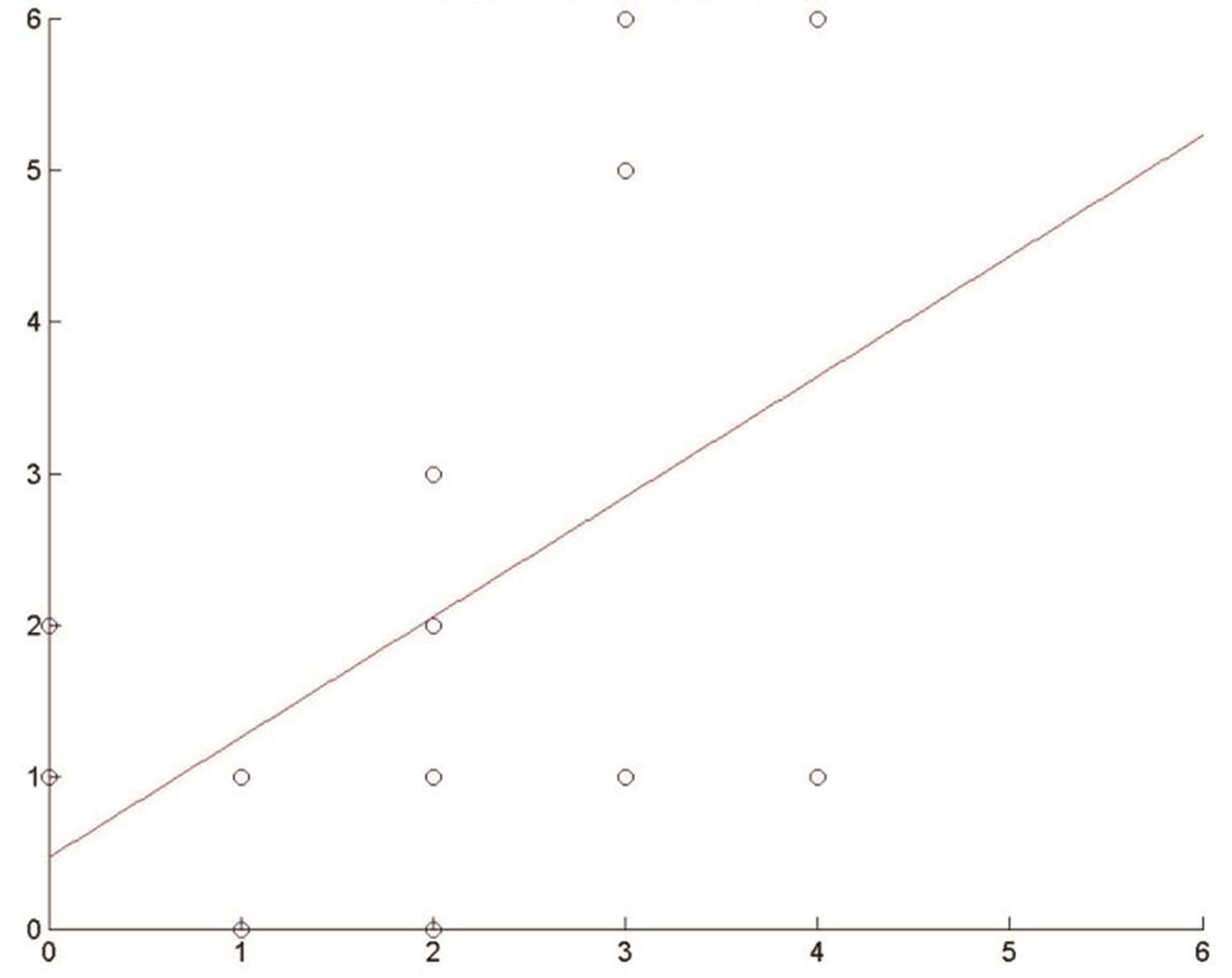
-
Fig. 4 Effect of intracerebral hemorrhage (ICH) score on modified Rankin Scale (mRS)s. Regression coefficient at 1 year.
Fig. 4 Effect of intracerebral hemorrhage (ICH) score on modified Rankin Scale (mRS)s. Regression coefficient at 1 year.
Hematoma Volume, Surgical Timing, and Outcome
In the “early surgery” subgroup, the average hematoma volume was 45.55 ± 23.21 mL, while in the “delayed surgery” subgroup was 29.89 ± 21.33 mL. As for the relationship between hematoma volume and outcome, patients with a volume <30 mL (standard cut-off volume used for the evaluation of ICH severity13) had an average mRS of 2.09 ± 1.13 at 3 months and of 1.54 ± 1.01 at 1 year. In contrast, patients with a volume >30 mL had an average mRS of 3.23 ± 1.31 at 3 months and of 2.38 ± 1.91 at 1 year.
We also calculated the regression coefficient of variables from here on (i.e., volume, age, GCS, infratentorial hemorrhage, IVH) in relation to outcome. We excluded ICH score, being this a synthesis of the other variables.
The following linear model was applied:

Regarding volume, we obtained the following coefficients: 0.02 at 3 months and 0.016 at 1 year, indicating that an increase in 1 mL in the hematoma volume relates to an “expected” increase of 0.020 at 3 months and of 0.016 at 1 year in terms of mRS.
GCS, Surgical Timing, and Outcome
Patients in the “early surgery” subgroup had an average GCS of 7.64 ± 2.86, while those in the “delayed” one had an average GCS of 13.21 ± 2.39.
As for the relationship between GCS and outcome, patients with a favorable mRS at 3 months had a debut average GCS of 11.93 ± 3.11 and those with a favorable mRS at 1 year had an average GCS of 11.36 ± 3.80. Patients with an unfavorable mRS at 3 months had an average GCS of 8.9 ± 2, 87 and those with an unfavorable mRS at 1 year had an average GCS of 8.6 ± 2, 81.
As described earlier, we also calculated for this variable regression coefficient against outcome with the following results: 0.196 at 3 months and 0.192 at 1 year, both negative. This means that an increase in 1 point in the GCS scale corresponds to a decrease of 0.196 at 3 months and of 0.192 at 1 year in terms of mRS.
Infratentorial Origin of Hemorrhage, Surgical Timing, and Outcome
Three patients (27.3%) in the “early surgery” subgroup had an infratentorial hemorrhage at onset. In the “delayed surgery” subgroup infratentorial hemorrhage was present in two patients (14.2%). Very interesting is the relationship with outcome. Patients with infratentorial bleeding presented an average mRS of 4 ± 1.65 at 3 months and of 3.4 ± 2.05 at 1 year. In contrast, patients without infratentorial hemorrhage presented an average mRS of 2.3 ± 0.96 at 3 months and of 1.6 ± 0.77 at 1 year. We have also achieved a linear regression coefficient of the variable “infratentorial origin” in respect of mRS of 1.84 and 1.89 at 3 months and 1 year, respectively, both positive: this means that infratentorial origin of hemorrhage entails an expected increase in mRS of 1.84 at 3 months and of 1.89 at 1 year, that is a clear worsening of outcome.
Presence of IVH, Surgical Timing, and Outcome
The presence of IVH does not seem to have affected surgical timing, being present in five patients (45.5%) in the “early surgery” subgroup and in six patients (42.82%) in the “delayed surgery” one. Furthermore, the study of population in relation to this aspect did not allow us to obtain clear results about the correlation with outcome.
Age, Surgical Timing, and Outcome
The average age of patients in the two groups was 38 ± 18 years in the “early surgery” subgroup and 41 ± 16 years in “delayed surgery” one: no statistically significant differences were, therefore, found. More interesting is the relationship between age and outcome. Patients aged <40 years had an average mRS of 2.14 ± 1.12 at 3 months and of 1.21 ± 0.75 at 1 year. Those aged >40 years had an average mRS of 3.27 ± 1.73 at 3 months and of 2.9 ± 1.65 at 1 year. Even more interesting is the difference Δ(mRS) (1 year vs. 3 months) between the two populations which that 0.92 ± 0.43 in patients aged <40 years and 0.36 ± 0.25 in patients aged >40 years. This may suggest that the potentiality of outcome improvement is greater in younger patients. Also here, we calculated the regression coefficient of the variable “age” compared with mRS, obtaining the following results: 0.04 and 0.05 at 3 months and 1 year, respectively; this means that the 1-year increase in age leads to an expected increase in mRS of 0.04 at 3 months and of 0.05 at 1 year.
Spetzler-Martin Grade, Surgical Timing, and Outcome
Nine patients in the “early surgery” subgroup (82%) harbored a Spetzler-Martin grade I or II AVM (class A). Only one patient (9%) had a grade III (class B) AVM. In the same way, only one patient (9%) had a grade IV AVM. Regarding treatments, in the patient harboring grade IV (class C) AVM the only evacuation of the hemorrhage was performed, while in the patient with grade III (class B) AVM treatment was multimodal (i.e., endovascular embolization and microsurgery resection).
As for the “delayed surgery” subgroup, 50% of the patients harbored a grade I or II AVM (class A, 7 patients), while 50% had a grade III AVM (class B, 7 patients). All grade III lesions were subjected to presurgical endovascular embolization (multimodal treatment), though in one case it was not possible to complete the procedure.
To understand the existing link between Spetzler-Martin grading and patient outcomes, the most homogeneous subgroup, that is, the “delayed surgery” one (50% grades I/II vs. 50% grade III), was selected; patients with class A AVM (grades I/II) had an average mRS of 1.71 ± 0.63 at 3 months and of 1 ± 0.42 at 1 year. Patients with class B AVM had an average mRS of 2 ± 1.22 at 3 months and of 1.56 ± 0.62 at 1 year. We also calculated the average ICH score of these two subgroups, and it was the same: 1.14 ± 0.83.
To identify the effect and the weight of the aforementioned parameters (Spetzler-Martin grade and ICH score) on outcomes, we used the same linear regression model applied above. Then we calculated the regression coefficient of both variables in relation to mRS, obtaining the following results: 0.44 for the ICH score and 0.29 for the Spetzler-Martin grade at 3 months; 0.25 for the ICH score and 0.57 for Spetzler-Martin grading at 1 year. This means that, even if in all cases the coefficients were positive and useful for the prediction of patient outcomes, mRS was most influenced by the ICH score (and so by the severity of hemorrhage) at 3 months; the situation was instead reversed at 1 year, being the outcome more influenced by Spetzler-Martin grade, and so by angioarchitectural AVM aspects and by their inherent surgical risk.
Discussion
Current neurosurgical literature still lacks randomized controlled trials14 showing that the benefits of the definitive treatment of bAVMs outweigh the risks. However, given that hemorrhage at diagnosis is one of the most relevant risk factors for subsequent new hemorrhage,3 definitive treatment is widely recommended,7 14 15 16 17 with the exception of those patients for which, because of the high surgical risk of the lesion (Spetzler-Ponce class C or Spetzler-Martin grades IV and V), conservative management is recommended.16 17 18
The outcome of patients with ruptured bAVMs is generally favorable, with a mortality rate and a permanent disability rate relatively low compared with those of spontaneous intracerebral hemorrhage (sICH).
International literature2 7 8 9 10 recommends, in general, a “rest period” (1–6 weeks) between the hemorrhage and the conclusive treatment of the disease, since the risk of early rebleeding, though possible, is rare, and the “parenchyma” of the lesion can be “friable” in the period following the hemorrhage thus increasing surgical risk. It has been stated that this approach allows the safest surgical treatment (when this is indicated), also through a better diagnostic evaluation of the problem. However, when (1) significant mass effect is present, or (2) it is not possible to stabilize the patient and conservatively control intracranial and cerebral perfusion pressures, with subsequent progressive neurological deterioration, an urgent evacuation of the hematoma may be necessary.10 19 Management of this situation may also include the early resection of the AVM in the same single surgical time. AVM definitive treatment should be considered for those injures that are not expected to have a high surgical risk8; in this sense, there are also studies showing that this management is associated with a very favorable outcome.9
In our study, we found that, in case of hemorrhagic bAVMs, ICH score evaluation may be decisive in the choice of the best surgical timing. ICH score can also be one main predictive factor for patient outcome (in particular for early outcome), in accordance with Hemphill et al.20 This finding suggests that ICH score, scarcely used in presence of hemorrhagic bAVMs, can be a useful tool for both decision-making regarding therapeutic management, and outcome prediction, especially when considered along with two of the other characteristics examined, that is, age and Spetzler-Martin grade.21 The ICH score parameters that seemed to influence surgical timing the most were GCS and hematoma volume.
As far as outcomes are concerned, regression coefficients allowed us to detect a significant correlation with GCS, hematoma volume, and infratentorial origin.
It has been extremely interesting to note that while GCS and hematoma volume seem to affect especially early outcomes, the presence of an infratentorial hemorrhage affects both early and late outcomes. Infratentorial origin of the hemorrhage should, therefore, be considered in the decision-making about surgical timing. Moreover, also sICH guidelines emphasize that, in the presence of a cerebellar hemorrhage determining neurological deficits or hydrocephalus from IV ventricle obstruction, the early evacuation should be considered.22
Age deserves a separate discussion, as it is a variable that strongly influences outcomes.
Patients under 40 years of age had a significantly better outcome than patients older than 40 years. This parameter may explain why the outcome of patients affected by intracranial hemorrhage from ruptured bAVM is usually better than that of patients affected by primary sICH, being these patients generally older. Accordingly, in a population-based study released in 2008, van Beijnum et al found that in case of AVM-related ICH, patients were younger, had a lower pre-stroke blood pressure, and a higher GCS at the entrance. They also were more likely to have an atypical ICH (lobar location) compared with patients affected by primary sICH.23
We have already argued about surgical risk determined by AVM characteristics.11 18 24
Certainly, also in our case, the grade of AVM influenced surgical timing. Nearly everyone within the “early surgery” group harbored a grade I or II AVM (Spetzler-Ponce class A), only one patient had a grade III and one patient a grade IV. Instead, 50% of patients in the “delayed surgery” group presented a grade III AVM (Spetzler-Ponce class B). Grade III AVMs are injuries for which current literature recommends a careful angiographic assessment and multimodal treatment, wherever possible.18
In our study, early outcomes seem more influenced by the ICH score and therefore from hemorrhage severity, while delayed outcomes by Spetzler-Martin grading; this confirms the role of AVM angioarchitectural anatomy in the preoperative surgical risk assessment with the purpose to guide surgeons in the choice of the best treatment possible.16 17 18 It is, so, actually preferable (when possible) to intervene on the malformation away from the hemorrhage, to assess the best therapeutic strategy.16 17 According to Lawton et al,17 if a patient would have an additional benefit from embolization (provided that the risk is tolerable), double modality management appears indicated; of course, if the risk associated with embolization seems too high (e.g., in case of elevated intracranial pressure) only microsurgery should be done. Our analysis also showed that patients under 40 years of age with poor neurological status at the entrance, average ICH score of 2.67 ± 0.62, and low AVM surgical risk, undergoing early resection of the lesion, had a 3-month poor average mRS (2.47 ± 0.71), while at 1 year the outcome was very favorable (1 ± 0.4). This suggests, in line with other studies,9 18 25 how in young patients with a high ICH score at the entrance, one or more risk factors consistently related to poor outcome (low GCS, volume clot > 30 mL, infratentorial hemorrhage), and a low-grade Spetzler-Martin malformation, early intervention of evacuation and AVM removal in one single surgical time represents a valid and feasible therapeutic strategy. In a study released in 2020, Deng et al reported a single-center study on 111 hemorrhagic bAVMs in children suggesting that early surgical treatment is preferable following the hemorrhagic presentation.25 Aboukaïs et al26 found that in grade I bAVMs long-term morbidity is, in the majority of cases, related to the brain damage determined by the hemorrhagic event; only in few cases, this may be linked to AVM resection.
Main limitations of this study are represented by the small cohort dimension, and by the retrospective fashion of the study.
Conclusions
When used together with Spetzler-Martin grading scale, ICH score seems to be a valid clinical instrument both in the management decision-making and in the outcome prediction in patients affected by hemorrhagic bAVMs. These parameters, considered together, allow a better evaluation about surgical timing, kind of treatment, and probable outcome.
Early outcomes seem to be more influenced by ICH score, and therefore by bleeding severity, delayed ones more by Spetzler-Martin grade, and so by AVM angioarchitecture. This suggests that it is indeed preferable, when possible, to intervene on the lesion itself after some time from the hemorrhage to evaluate the best treatment strategy.
Our study contributes to affirm the importance of an early treatment in patients affected by grade I or II bAVMs though with poor neurological status at the entrance. In fact, in young patients with a high ICH score at the entrance, one or more risk factors consistently related to poor outcome (low GCS, volume clot > 30 mL, infratentorial hemorrhage), and a low-grade Spetzler-Martin malformation, early intervention of evacuation, and AVM removal in one single surgical time represent a valid and feasible therapeutic strategy. Confirmation of this will, of course, require further study and larger patient cohorts.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
None declared.
Funding None.
References
- Natural history of arteriovenous malformations: presentation, risk of hemorrhage and mortality. Acta Neurochir Suppl (Wien). 2010;107:65-69.
- [Google Scholar]
- Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350-1355.
- [Google Scholar]
- Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58(3):331-337.
- [Google Scholar]
- Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065-1068. (9084)
- [Google Scholar]
- Arteriovenous malformations of the brain. A long-term clinical study. J Neurosurg. 1972;37(5):562-570.
- [Google Scholar]
- Special Writing Group of the Stroke Council, American Stroke Association. AHA Scientific Statement: recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 2001;32(6):1458-1471.
- [Google Scholar]
- Management of ruptured brain arteriovenous malformations. Curr Atheroscler Rep. 2012;14(4):335-342.
- [Google Scholar]
- Early surgery for ruptured cerebral arteriovenous malformations. Acta Neurochir Suppl (Wien). 2005;94:111-114.
- [Google Scholar]
- Arteriovenous malformation-associated intracerebral hemorrhage. World Neurosurg. 2012;78(6):586-587.
- [Google Scholar]
- The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34(1):2-6, discussion 6–7.
- [Google Scholar]
- Reliability of the modified Rankin Scale across multiple raters: benefits of a structured interview. Stroke. 2005;36(4):777-781.
- [Google Scholar]
- American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060.
- [Google Scholar]
- Interventions for treating brain arteriovenous malformations in adults. Cochrane Database Syst Rev (7):CD003436.
- [Google Scholar]
- Intracerebral hemorrhage: external validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29(6):658-663.
- [Google Scholar]
- Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(8):e200-e224.
- [Google Scholar]
- A 3-tier classification of cerebral arteriovenous malformations. Clinical article. J Neurosurg. 2011;114(3):842-849.
- [Google Scholar]
- Selection of treatment modalities or observation of arteriovenous malformations. Neurosurg Clin N Am. 2012;23(1):63-75.
- [Google Scholar]
- The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891-897.
- [Google Scholar]
- Predicting outcome after arteriovenous malformation-associated intracerebral hemorrhage with the original ICH score. World Neurosurg. 2012;78(6):646-650.
- [Google Scholar]
- Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- [Google Scholar]
- Outcome after spontaneous and arteriovenous malformation-related intracerebral haemorrhage: population-based studies. Brain. 2009;132:537-543. (Pt 2)
- [Google Scholar]
- Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33(7):1816-1820.
- [Google Scholar]
- Long-term outcomes and prognostic predictors of 111 pediatric hemorrhagic cerebral arteriovenous malformations after microsurgical resection: a single-center experience. Neurosurg Rev. 2020;•••
- [CrossRef] [Google Scholar]
- Grade 1 Spetzler and Martin cerebral ruptured arteriovenous malformations treated by microsurgery: Poor functional outcome is related to injury from haemorrhage. Neurochirurgie. 2017;63(2):69-73.
- [Google Scholar]






