Translate this page into:
Role of Biomarkers in Differentiating New-onset Seizures from Psychogenic Nonepileptic Seizures
Address for correspondence: Dr. Mahendra Javali, Department of Neurology, M. S. Ramaiah Medical College, New BEL Road, Bengaluru - 560 040, Karnataka, India. E-mail: mahendrajv@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Review of literature revealed very limited studies considering a combination of serum prolactin (PRL) and serum creatine kinase (CK) as markers for differentiating epileptic and psychogenic nonepileptic seizures (PNES). Therefore, in the present study, we analyzed the role of serum PRL and serum CK, individually and in combination.
Methodology:
This prospective study was conducted in a tertiary care medical teaching hospital over a period of 18 months. Patients aged over 15 years suspected to have new-onset seizures presenting within 5 h of ictus were included in this study. CK, serum PRL was measured at 0–1, 1–3, and 3–5 h after seizures.
Results:
Hundred subjects were studied for the role of serum PRL and serum CK in differentiating epileptic and PNES. The mean age was 42.24 years with a male:female ratio of 1.27:1. All patients of generalized tonic–clonic seizures (GTCS), who presented within 1 h, had elevated PRL, whereas 75% of patients with partial seizures had elevated PRL within 1 h of presentation. Nearly 91.66% of patients with GTCS who presented within 1 h had elevated CPK, whereas 70% of patients with partial seizures had elevated CPK. None of the patients diagnosed with PNES showed rise in either of the markers.
Conclusion:
In the present study, none of the patients with PNES showed raise in either serum PRL or CK. However, there was no correlation between the types of seizure and PRL or serum CK levels.
Keywords
Biomarkers
creatine kinase
psychogenic nonepileptic seizures
prolactin
seizures
INTRODUCTION
A seizure represents the clinical expression of abnormal, excessive, synchronous discharges of neurons residing primarily in cerebral cortex. Epilepsy considered to be a disease of the brain defined by any of the following conditions: (1) At least, two unprovoked (or reflex) seizures occurring >24 h apart; (2) one unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; (3) diagnosis of an epilepsy syndrome.[1] Psychogenic nonepileptic seizures (PNES) are defined as change in behavior or consciousness resembling epileptic seizures but which have a psychological origin. PNES are categorized as a manifestation of dissociative or somatoform (conversion) disorders. Video-electroencephalogram (EEG) recording of an event is the gold standard for the diagnosis.[2] The diagnosis of PNES relies on a multidisciplinary evaluation and is usually based on the different combinations of data. Recording a seizure while under video-EEG monitoring, is the most reliable diagnostic test. However, not all patients present with seizures while under video-EEG monitoring. Furthermore, not all epileptic seizures produce visible changes in the scalp EEG. The incidence of PNES was estimated to be 1.4–4.9/100,000/year in three previous studies, and the prevalence was calculated to be between 2 and 33/100,000 in one study, making it a significant neuropsychiatric condition.[3] However, by the time, these patients are diagnosed correctly, they would have been on antiepileptic medications for years. This not only contributes to unnecessary healthcare costs but also a wrong diagnosis exposes the patient for long-term adverse effects of anticonvulsant drugs, expense of therapy, and above all social implications, which makes it essential for accurate diagnosis, before starting the treatment. Serum prolactin (PRL) and creatine kinase (CK) have been traditionally studied to differentiate epileptic seizures from PNES while the initial studies showed promising results; they were criticized for many weaknesses in the study design. Given that these tests are readily available in all settings and cost-effective, it would be worthwhile to rekindle the work in this field.
Review of literature revealed very limited studies considering combination of serum PRL and serum CK as markers for differentiating epileptic and nonepileptic seizures. Therefore, in the present study, we analyzed the role of serum PRL and serum CK, individually and in combination, in relation to differentiate epileptic and PNES.
METHODOLOGY
This prospective study was conducted in a tertiary care medical teaching hospital over a period of 18 months. The Institution's Ethical Committee approved the study.
Patients aged over 15 years suspected to have new-onset seizures presenting within 5 h of ictus were included in the study. Known epileptics, pregnant women, patients with comorbidities such as renal and endocrine disorders and those who had received any intramuscular injections just before arrival at our setting were excluded from the study.
On arrival, a careful and detailed history was recorded, and thorough examination was conducted. Blood examinations which included complete blood count (hemoglobin, total leukocyte count, platelet count), erythrocyte sedimentation rate, renal function test (serum creatinine, blood urea nitrogen), liver function test (total/direct/indirect), alanine aminotransaminase, aspartate transaminase, gamma-glutamyl transpeptidase, serum electrolytes (serum sodium, serum potassium, serum chloride, and serum magnesium) were done on admission to rule out any comorbidities that could bias the study.
Total leukocyte count, CK, serum PRL were measured at 0–1, 1–3, and 3–5 h after seizures. Chest X-ray, electrocardiography, and EEG were done. Cerebral imaging (computerized tomography and/or magnetic resonance imaging) was also done to rule out any organic etiologies of the seizure.
Serum PRL was measured using an automated machine using ADVIA Centaur CP Readypacks (Bayer Healthcare LLC, USA). The ADVIA Centaur CP PRL assay is a two-site sandwich immunoassay using direct chemiluminometric technology, which uses constant amounts of two antibodies. The first antibody is a lite reagent, a polygonal goat anti-PRL antibody labeled with acridinium ester. The second antibody, in the solid phase, is a monoclonal mouse anti-PRL antibody, which is covalently coupled to paramagnetic particles. 25 μl of serum is incubated with 100 μl of lite reagent in a cuvette at 37°C for 6.3 min. This mixture is then incubated with 450 μl of solid phase for 3 min at 37°C. The cuvette is then washed, and acid and base reagents are added into the cuvette to initiate the chemiluminescent reaction. A direct relationship exists between the amount of PRL in the serum and the amount of relative light units detected by the automated system.
CK was measured using Dimension® clinical chemistry system (Dade Behring Inc., USA), an in vitro diagnostic test intended for the quantitative determination of CK activity in serum and heparinized plasma. The principle of the procedure is a modification of ultra-violet enzymatic determination described by Oliver and Rosalki,[45] CK catalyzes the transphosphorylation of phosphate from creatine phosphate to adenosine-diphosphate producing adenosine-triphosphate (ATP). The ATP thus formed is used to phosphorylate glucose in a reaction catalyzed by hexokinase, and the resulting glucose-6-phosphate is oxidized by glucose-6-phosphate dehydrogenase with the simultaneous reduction of nicotinamide adenine dinucleotide phosphate (NADP). The change in absorbance at 340 nm due to the formation of NADP is directly proportional to the CK activity since other reactants are present in nonrate limiting quantities and are measured using a bichromatic (340, 405 nm) rate technique.
Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences for Windows 16.0 (SPSS Inc., Chicago, IL, USA). The results for each parameter (numbers and percentages) for discrete data and average (mean ± standard deviation) for continuous data are presented in tables and figures using Microsoft Office 2007 software package.
Student's t-test was performed to assess the significance of serum PRL and total CPK with epileptic and nonepileptic seizures. A value of P < 0.05 was considered statistically significant.
RESULTS
Hundred subjects were studied for the role of serum PRL and serum CK in differentiating epileptic and PNES.
The mean age was 42.24 years with a male:female ratio of 1.27:1.
Thirty-six percent of the patients presented with generalized tonic–clonic seizures (GTCS). The next common presentation was simple partial seizures (29%), complex partial seizures (CPS) (19%), and status epilepticus (SE) (7%) at admission. Nine percent of patients were retrospectively diagnosed to have PNES using video-EEG.
All patients with GTCS, who presented within 1 h had elevated PRL, whereas 75% of patients with partial seizures had elevated PRL within 1 h of presentation [Table 1].
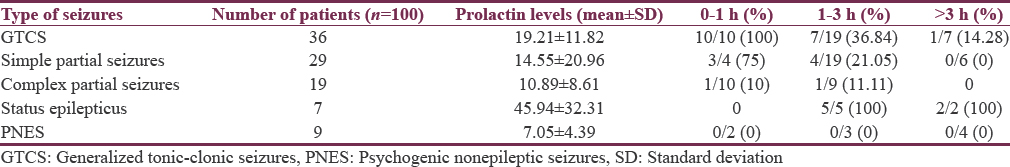
Nearly 91.66% of patients with GTCS who presented within 1 h had elevated CPK whereas 70% of patients with partial seizures had elevated CPK [Table 2].
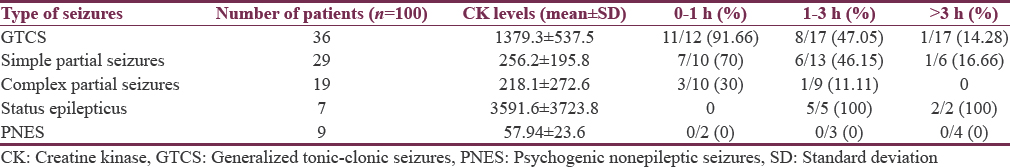
None of the patients diagnosed with PNES showed rise in either of the markers.
All patients with GTCS, who presented within 1 h, had elevated PRL whereas 75% of patients with partial seizures had elevated PRL within 1 h of presentation.
Nearly 91.66% of patients with GTCS who presented within 1 h had elevated CPK whereas 70% of patients with partial seizures had elevated CPK.
DISCUSSION
PRL release from the pituitary is controlled by the hypothalamus through a PRL inhibitory factor, now believed to be dopamine,[6] it is possible that the generalized neuronal discharge of a seizure stimulates the hypothalamus either directly, through specific neurotransmitter changes, or through the release of other substances.[7]
Serum PRL has been tried and tested in the recent past as a marker to differentiate epileptic and nonepileptic seizures. Kurlemann et al. reported 2- to 3-fold increase in PRL levels in patients after either generalized seizures or CPS, but not after psychogenic seizures.[8]
Our study confirms the previous observation that serum PRL increases following generalized seizures. All the ten patients (100%) who presented with in first hour of GTCS had elevated PRL levels. Only 37% of patients with GTCS who presented between 1 and 3 h after seizure had elevated PRL, whereas, only 14% of patients who presented after 5 h had elevated PRL.
Our findings were comparable to the findings of various study groups,[910] who concluded that if PRL was measured 10–20 min after an event, then it probably can be a useful measure to differentiate between a generalized tonic–clonic seizure and PNES. However, if the serum PRL test is taken 5 h after the event, then it is probably indicative of the baseline PRL level of that patient.
A serum PRL test is not recommended in the routine assessment of a possible seizure. There are several limitations to using postictal serum PRL elevation:
-
It cannot be used to differentiate simple partial seizures or absence seizures from nonepileptic seizures
-
PRL levels may increase following vasovagal syncope, and elevations have been observed following tilt table testing
-
CPS that do not arise from the temporal lobe do not lead to PRL elevation
-
A postictal PRL rise may not be shown by 10%–20% of patients with tonic-clonic seizures
-
Ambiguous test results, such as a 2-fold elevation, are difficult to interpret. PRL level rises predictably only after a single seizure. Patients who have more than two seizures in 12 h have progressively smaller elevations, presumably because stored PRL from the pituitary lactotrophs is exhausted.[71112]
The mean PRL level in patients with generalized seizures was 28.6 ng/ml compared to 10.4 ng/ml (P < 0.001) in patients with pseudoseizures in a study done by Singh and Jana.[13]
Libman et al. previously reported that serum CK is highly specific for diagnosing generalized seizures with improved sensitivity by sampling serum at least 3 h postictally,[14] Chesson et al. revealed that postictal elevation of serum CK appears to be related to the intensity of muscular activity,[15] the magnitude of elevation of serum CK is a more sensitive indicator of GTC epileptic seizures than its absolute level, and that this elevation occurs late postictally. Neufeld et al. concluded that a high increase of CK levels occurs in the 2nd day in GTC seizures, even when sequential tests are within the normal range; they added that an increase of at least 15 U/L is highly indicative of an epileptic event.[16]
In our study, 91.66% of patients with GTCS who presented within 1 h had elevated total CPK levels whereas only 47% of patients who presented between 1 and 3 h had elevated total CPK levels. Seventy per cent of patients with partial seizure who presented within 1 h had elevated total CPK levels whereas 46% of patients who presented between 1 and 3 h had elevated total CPK levels. All patients with SE had elevated total CPK levels irrespective of the time interval from onset of seizure.
No patient of PNES had elevated total CPK level.
Our results showed that the serum CPK level was a specific indicator of a recent convulsive seizure. Studies suggest that the presence of high serum CPK values correlates strongly with the presence of a seizure as the cause of loss of consciousness. This test has high specificity but only moderate sensitivity.[1517] Minor trauma appears to have little effect on these results, but false positives may occur in the setting of undiagnosed myocardial infarctions, intramuscular injections.
CONCLUSION
In the present study, none of the patients with PNES showed raise in either serum PRL or CK. However, there was no correlation between the types of seizure and PRL or serum CK levels. Our findings were partially in line with the findings with the rest of world literature that there was no relation of raised markers and type of seizure. Yet, an absence of raised serum PRL and serum CK levels can be used to differentiate PNES from epileptic seizures owing to their individual strong negative predictive value.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- ILAE official report: A practical clinical definition of epilepsy. Epilepsia. 2014;55:475-82.
- [Google Scholar]
- A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J. 1955;61:116-22.
- [Google Scholar]
- An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967;69:696-705.
- [Google Scholar]
- The hormonal responses to generalized tonic-clonic seizures. Brain. 1984;107(Pt 2):569-78.
- [Google Scholar]
- Serum prolactin after cerebral and psychogenic seizures in childhood and adolescence – An additional useful method for differentiating the two forms of seizure. Klin Padiatr. 1992;204:150-4.
- [Google Scholar]
- Serum neuron-specific enolase, prolactin, and creatine kinase after epileptic and psychogenic non-epileptic seizures. Acta Neurol Scand. 2004;109:318-23.
- [Google Scholar]
- Capillary prolactin measurement for diagnosis of seizures. Ann Neurol. 1991;29:187-90.
- [Google Scholar]
- Endocrine aspects of partial seizures. In: The Comprehensive Evaluation and Treatment of Epilepsy. A practical guide Elsevier; 1997. p. :207-32.
- [Google Scholar]
- Serum prolactin and cortisol in children with some paroxysmal disorders. Indian J Pediatr. 1994;61:57-61.
- [Google Scholar]
- Seizure vs. syncope: Measuring serum creatine kinase in the emergency department. J Gen Intern Med. 1991;6:408-12.
- [Google Scholar]
- Sequential serum creatine kinase determination differentiates vaso-vagal syncope from generalized tonic-clonic seizures. Acta Neurol Scand. 1997;95:137-9.
- [Google Scholar]
- Postictal serum creatine kinase in the diagnosis of seizure disorders. Arch Neurol. 1985;42:123-6.
- [Google Scholar]






