Translate this page into:
Revisiting the tubercular zone: A poor prognostic finding on neuroimaging
*Corresponding author: Himanshu Kaushal, Department of Neurology, Mahatma Gandhi Medical College and Hospital, RIICO Institutional Area, Jaipur, Rajasthan, India. himanshukaushal1993@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kaushal H, Goyal G. Revisiting the tubercular zone: A poor prognostic finding on neuroimaging. J Neurosci Rural Pract. 2024;15:370-2. doi: 10.25259/JNRP_476_2023
Abstract
Central nervous system tuberculosis accounts for approximately 1–2% of cases but with a high morbidity and mortality burden. A 37-year-old female presented with fever and headache for 15 days followed by altered sensorium with associated dystonic posturing of both upper limbs and lower limbs (left>right side). The patient’s condition deteriorated despite optimal antitubercular treatment and other supportive measures for two weeks. An MRI brain was suggestive of areas of diffusion restriction in the right caudate nucleus, anterior limb of internal capsule, genu, and anteromedial thalamus. The patient ultimately succumbed to death. Tubercular zone infarctions carry an ominous prognosis and can be considered an indicator of morbidity and mortality in patients with tuberculous meningitis (TBM).
Keywords
Tubercular zone
Ischemic zone
Central nervous system tuberculosis
Tuberculous meningitis
Palur grade
INTRODUCTION
Tuberculosis (TB) caused by Mycobacterium tuberculosis (MTB) is one of the most common infectious diseases of developing countries. As per World Health Organization (WHO) global TB report 2022, the incidence of TB had increased from 10.1 million in 2020 to 10.6 million in 2021with ~15.1% mortality.[1] Primary infection with TB evokes a protective response whereas post-primary TB evokes a destructive response. When the host has sufficient immunity, primary TB occurs where granulomas form. Dissemination produces a whole clinical spectrum of meningitis, miliary tuberculosis, renal and adrenal TB, third space effusions, abscesses, etc.[2] Central nervous system tuberculosis (CNS TB) accounts for ~1–2% of cases but with significant morbidity and mortality burden. Most common presentation of CNS TB is tuberculous meningitis (TBM). Furthermore, encephalitis, tuberculoma/s, vasculitis, ventriculitis, myelitis, or tuberculous abscess can be seen.[3] Infarcts are not uncommon in TBM. Hsieh et al., in 1992, first described the tubercular zone (also known as TB zone), an area supplied by the medial lenticulostriate and thalamoperforating arteries. In contrast, the ischemic zone is the area supplied by the lateral lenticulostriate, anterior choroidal, and thalamo-geniculate arteries.[4] The “TB zone” involves (1) head of the caudate nucleus, (2) genu of the internal capsule, (3) the anterior limb of internal capsule, and (4) the anteromedial segment of the thalamus, whereas lentiform nucleus, posterior limb of the internal capsule, and posterolateral segment of thalamus contribute to the “Ischemic zone.”[5]
CASE REPORT
A 37-year-old female presented with fever and headache for 15 days followed by altered sensorium in the form of drowsiness and was admitted with a working diagnosis of febrile encephalopathy. Initial contrast-enhanced MRI brain [Figure 1] was suggestive of pachymeningitis. Cerebrospinal fluid (CSF) study revealed a normal opening pressure of 24 cm of water. Microscopy was predominately lymphocytic (55 cells). The CSF TB polymerase chain reaction (PCR) was positive. During the hospital stay, the patient had a sudden worsening of sensorium characterized by a fall in the Glasgow Coma Scale (GCS) score and dystonic posturing of both upper limbs and lower limbs (left> right side). The patient had deterioration in condition despite of optimal antitubercular treatment and other supportive measures for two weeks. The repeat MRI [Figure 2] was suggestive of areas of diffusion restriction in the right caudate nucleus, anterior limb of the internal capsule, genu, and anteromedial thalamus (as shown in figures). The patient was started on aspirin and steroids along with antitubercular therapy in view of infective vasculitis-related infarction; however, the patient did not improve remarkably.

- Magnetic resonance imaging brain (axial T1-weighted post-gadolinium) suggestive of diffuse pachymeningeal enhancement more prominent on the left frontotemporal regions.
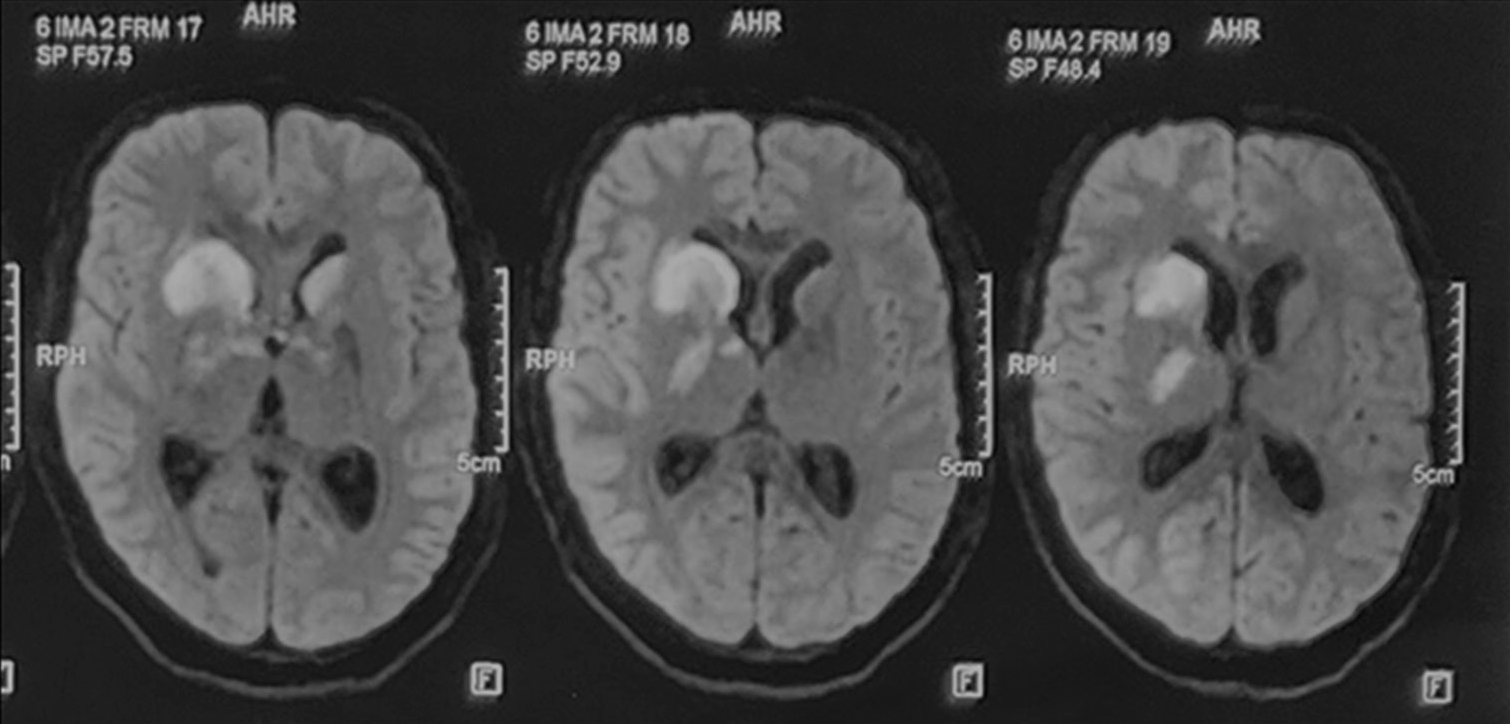
- The magnetic resonance imaging brain diffusion-weighted images showing areas of diffusion restriction in “the tubercular zone” in head of caudate nucleus, anterior limb, and genu of internal capsule, anteromedial thalamus on the right and head of caudate nucleus on the left side after worsening of sensorium.
The patient had further deterioration in condition. The MR venogram and repeat CSF examination (including cryptococcal, fungal stain, malignant cytology, and encephalitis panel) were unremarkable. Her serum angiotensin-converting enzyme (ACE) levels and immunoglobulin G4 (IgG4) levels were also within normal limits. Her pupils were 4 mm dilated, not reacting to light. Corneal and conjunctival reflexes were sluggish. Doll’s eye response was absent. Electroencephalograph was suggestive of diffuse slowing of background activity in 3–4 Hz 30–40 µV delta frequency range. The patient ultimately succumbed to death despite optimal medical management.
DISCUSSION
Stroke incidence in TBM varies from 15% to 67%, and it is mostly associated with poor outcome.[6] Infarction patterns have been described in tubercular meningitis in the past. Literature has focused on infarcts in the TB zone as poor prognostic sign. In the present case, the subcortical location of infarcts in the TB zone heralded a poorer prognosis. As discussed previously, Hsieh et al. studied the location of infarcts in patients in TBM. They observed that 75% of infarctions occurred in the “TB zone” whereas only 11% occurred in the “Ischemic zone,” and bilaterally symmetrical infarctions were seen more commonly in TB zone (71%),[5]although in the present case, the infarctions in TB zone were asymmetrical. Tai et al. (2016) concluded that 67% of patients withTBM had cerebral infarction. Classifying the infarctions as patterns discussed by Hsieh et al., 20 out of 34 patients (59%) had infarcts in both “TB zone” and “ischemic zones.” On the other hand, 12 patients (35%) and 2 patients (6%) had infarcts in “ischemic zone” and “TB zone,” respectively.[4] Soni et al. in 2019 studied 90 TBM patients. About 57.7% patients developed cerebral infarcts. Among these infarctions, 35%, 13%, and 15% occurred in ischemic, tubercular, and both zones, respectively. Our case lacked involvement of the ischemic zone. The vascular involvement in TBM ranges from microscopic to macroscopic involvement. Small, medium, and large vessels can be involved leading onto different presentations such as subcortical lacunar infarcts and large vessel occlusions. It has been seen that direct vessel wall damage by MTB, immunological destruction of the vessel wall as seen in vasculitis, and accelerated atherosclerosis and ultimately thrombogenesis can be the contributory factors for the development of stroke in patients with TBM. In the same study, poorer outcome was associated with overall severity of illness, grade of TBM, stroke association, and burden of cerebral infarctions.[7] In the present case, illness was severe in the form of poorer GCS and FOUR score, Palur grade IV that is deeply comatose; however, the burden of infarctions was asymmetrical involving the basal ganglia structures that were an indicator of poor prognosis. The pattern of infarctions was of small vessel type. Latest systematic review published in 2022, 71 studies were included in the review with a total of 2194 patients with TBM, who developed stroke. The estimated frequency of stroke in TBM was found to be 0.30 (95% confidence interval, 0.26– 0.33). Fever and headache were the two most common clinical symptoms at presentation, and basal ganglia, cerebral cortex, and internal capsule were most frequently involved. The pooled proportions of mortality and poor outcomes were 0.22 and 0.51, respectively.[8] The main limitation of this case report is being a single case. The results and findings of the same cannot be generalized to a large cohort of TBM patients. A significant sample size is needed to ascertain the prognostic signs in patients with TBM and to assess the role of neuroimaging findings as prognostic markers in TBM.
CONCLUSION
Infarction pattern in TBM can aid in prognostication. The overall stage of TBM, rapidity of deterioration, response to pharmacotherapy along with CSF parameters, and MRI findings are other important indicators of outcome and prognosis. Tubercular zone infarctions carry ominous prognosis and can be considered indicators of morbidity and mortality in patients with TBM.
Ethical approval
The research/study approved by the Institutional Review Board at Mahatma Gandhi Medical College and Hospital, Jaipur, Rajasthan, India, number MGMC&H/IEC/ JPR/2023/1663, dated 18-08-2023.
Declaration of patient consent
This was a retrospective case study hence consent was not required.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- WHO's global tuberculosis report 2022. Lancet Microbe. 2023;4:e20.
- [CrossRef] [PubMed] [Google Scholar]
- The pathogenesis of tuberculosis: The early infiltrate of post-primary (Adult Pulmonary) tuberculosis: A distinct disease entity. Front Immunol. 2018;9:2108.
- [CrossRef] [PubMed] [Google Scholar]
- Central nervous system tuberculosis: Pathogenesis and clinical aspects. Clin Microbiol Rev. 2008;21:243-61.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral infarction pattern in tuberculous meningitis. Sci Rep. 2016;6:38802.
- [CrossRef] [PubMed] [Google Scholar]
- Locations of cerebral infarctions in tuberculous meningitis. Neuroradiology. 1992;34:197-9.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke in tuberculous meningitis. J Neurol Sci. 2011;303:22-30.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebrovascular complications in tuberculous meningitis-a magnetic resonance imaging study in 90 patients from a tertiary care hospital. Neuroradiol J. 2020;33:3-16.
- [CrossRef] [PubMed] [Google Scholar]
- Global frequency and clinical features of stroke in patients with tuberculous meningitis: A systematic review. JAMA Netw Open. 2022;5:e2229282.
- [CrossRef] [PubMed] [Google Scholar]






