Translate this page into:
Remote multiple intracranial hemorrhage in multiple metastatic lung adenocarcinoma following decompression of posterior fossa lesion: Unknown cause
Address for correspondence: Dr. Dhaval P. Shukla, Department of Neurosurgery, Faculty Block, National Institute of Mental Health and Neurosciences, Bengaluru - 560 029, Karnataka, India. E-mail: neurodhaval@rediffmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cerebral metastasis can present with hemorrhage. However, multiple hemorrhages in metastatic lesions following surgical decompression of a single lesion are never reported. We report a case of cerebral metastasis from lung cancer that developed multiple hemorrhages in supratentorial metastatic lesions following surgical resection of an infratentorial lesion.
Keywords
Brain metastasis
external ventricular drain
lung cancer
tumor with bleed
Introduction
Intracranial hemorrhage in the metastatic lesion is a known phenomenon. However, multiple hemorrhages in supratentorial mass following decompression of infratentorial mass is not reported. Sudden decompression of infratentorial mass may have caused a sudden pressure difference between supratentorial and infratentorial compartment resulting in tumor microvasculature rupture causing a bleed in all metastatic lesions. We report a case of multiple intracranial bleed following decompression of posterior fossa component.
Case Report
A 60-year-old man presented with a headache suggestive of raised intracranial pressure for 2 months associated with vomiting and blurring of the vision at the peak of a headache. There was no past history suggestive of any primary malignancy in the body. There was no history suggestive of any genetic coagulopathy through detailed evaluation was not done. On examination, his blood pressure was 130/80 mmHg. He had bilateral papilledema and left side cerebellar signs; there were no other neurological deficits. Computer tomography (CT) of the head showed multiple intracranial mass lesions, solid, and cystic with enhancement following contrast administration. Magnetic resonance imaging of the brain showed similar findings [Figure 1]. There was no imaging evidence of hemorrhage with either modality of imaging. Chest X-ray showed right lower zone ill-defined opacity. Positron emission tomography scan showed multiple intracranial mass lesions, hypermetabolic soft tissue lesion in the right lung, and multiple mediastinal lymph nodes. His preoperative platelet count was 2.48 lakhs/mm3. The patient underwent right frontal external ventricular drain (EVD) placement followed by left paramedian suboccipital craniectomy and excision of the cerebellar lesion. There was around 450 ml of blood loss during surgery, and no blood/blood product transfusion was given during the surgery. After surgery, the patient had delayed reversal from anesthesia. CT scan of head immediately after surgery showed multiple bleeds in supratentorial mass lesions [Figure 2]. There were no petechiae, ecchymosis, or other hemorrhagic manifestations. Postoperative coagulation profile was normal (international normalized ratio - 1.2, activated thromboplastin time - 35 s, and platelet count - 2.37 lakhs/mm3). The patient was managed aggressively in Intensive Care Unit for reduction of intracranial pressure. The patient improved gradually and discharged. Postoperative coagulation profile revealed normal platelet count, bleeding time and clotting time. Histopathology report revealed metastatic adenocarcinoma; there was no evidence of bleed on histopathology.
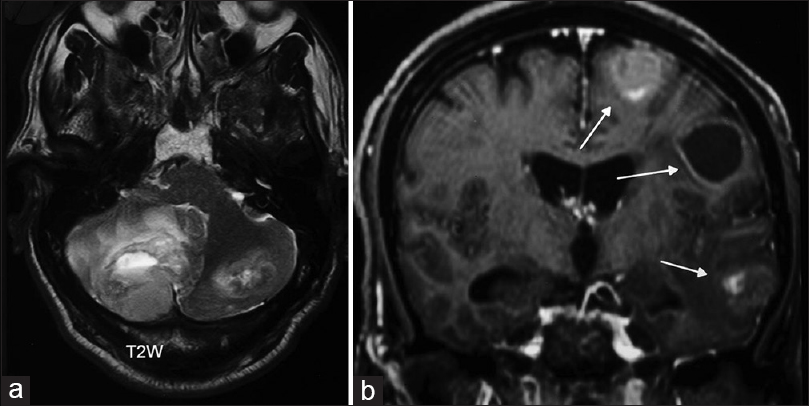
- Magnetic resonance imaging of the brain, (a) T2-weighted axial acquisition showing a large cerebellar tumor with edema and fourth ventricle compression. (b) T1-weighted following contrast administration is showing multiple metastatic lesions in the left cerebral hemisphere, solid, and cystic
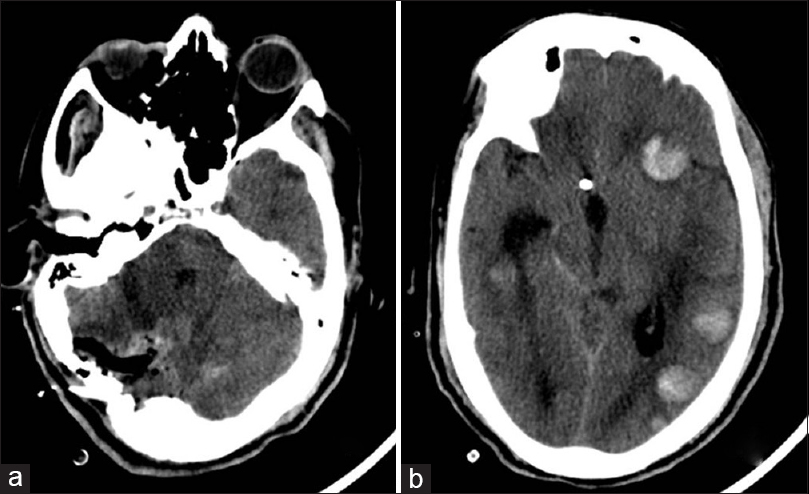
- Computer tomography scan of the head, (a) Axial section of posterior fossa showing postoperative changes. (b) Axial section of supratentorial brain showing hemorrhages in multiple lesions in the left hemisphere
Discussion
Primary lung adenocarcinoma has more incidence of brain metastasis than other carcinomas.[1] Hemorrhage into brain metastases is a fatal complication associated with significant morbidity and mortality. The incidence of spontaneous bleeding in lung metastases is l% and in breast carcinoma is 5% compared with significantly higher rates with thyroid cancer, melanoma (40–50%), renal cell cancer (70%), and choriocarcinoma.[2]
The mechanisms of bleed in metastatic lesion are endothelial proliferation with vascular obliteration, vessel compression and/or distortion due to rapid tumor growth, vessel necrosis, invasion of vessel walls by the tumor, and increased venous pressure associated with increased intracranial pressure.[34] Overexpression of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP) may play a role in metastatic brain tumor-associated hemorrhage than the nonhemorrhagic lesion.[5] The MMP-2 and MMP-9 specifically attack type IV collagen, laminin, and fibronectin, the major components of the basal lamina around cerebral blood vessels. As a result of degradation of extracellular matrix components of the basal lamina, MMP may play a major role in the loss of microvascular integrity leading to hemorrhagic transformation.[5] Overexpression of VEGF in tumor cells enhances tumor growth and metastasis by stimulating neovascularization, thereby increasing microvessel density. Higher levels of VEGF are found in hemorrhagic pleural fluids of patients with lung cancer.[6] Common histological features of tumors that bled include tumor necrosis, as well as the vascular changes of vessel wall hyalinization, degeneration or necrosis of vessel walls, thrombosis, the presence of many thin-walled vessels, and ruptured vessels.[7]
Hemorrhage within cerebral metastasis following cerebrospinal fluid (CSF) diversion has been reported.[3] The mechanism is a sudden change of pressure between supratentorial and infratentorial or upward herniation in posterior fossa lesion resulting in spontaneous hemorrhage within the lesion.[3] However, multiple hemorrhages in all supratentorial metastatic lesions has not been described in the literature. We proposed that following decompression of posterior fossa lesion, there was a sudden change of intracranial pressure resulting in bleed in all lesions. However, the possibility of the EVD leading to sudden intracranial hypotension may have also caused hemorrhages in our patient. Such complication may be reduced by slow release of CSF.
Conclusion
Multiple intratumor hemorrhage following posterior fossa decompression in a case of multiple intracranial metastases is a rare cause of neurological deterioration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698-705.
- [Google Scholar]
- What is the risk of intracranial bleeding during anti-VEGF therapy? Neuro Oncol. 2008;10:624-30.
- [Google Scholar]
- Fatal intratumoral hemorrhage in posterior fossa tumors following ventriculoperitoneal shunt. J Clin Neurosci. 2009;16:135-7.
- [Google Scholar]
- Significance of hemorrhage into brain tumors: Clinicopathological study. J Neurosurg. 1987;67:852-7.
- [Google Scholar]
- Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor-associated intracerebral hemorrhage. J Neurooncol. 2006;76:257-63.
- [Google Scholar]
- High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology. 2002;63:70-5.
- [Google Scholar]
- Surgical outcomes of hemorrhagic metastatic brain tumors. Cancer Res Treat. 2011;43:102-7.
- [Google Scholar]






