Translate this page into:
Recurrent hemodynamic ischemic strokes as the presenting feature of moyamoya disease
*Corresponding author: Yudhi Adrianto, Department of Neurology, Airlangga University, Faculty of Medicine, Airlangga University Hospital, Surabaya, Indonesia. yudhi_neuro@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Ashari Q, Adrianto Y, Setyowatie S. Recurrent hemodynamic ischemic strokes as the presenting feature of moyamoya disease: A case report. J Neurosci Rural Pract. doi: 10.25259/JNRP_86_2025
Abstract
Moyamoya disease (MMD) is a progressive and occlusive cerebrovascular disorder affecting the circle of Willis and surrounding arteries, characterized by a “puff of smoke” appearance on angiography. Recurrent transient ischemic attacks (TIAs) and strokes are common manifestations, frequently attributed to impaired cerebral hemodynamics due to poor collateral blood flow. This hemodynamic compromise A 34-year-old woman presented with four episodes of right-sided hemiparesis, slurred speech, and intermittent headaches over eight months, each resolving spontaneously within several days. Neuroimaging, including a non-contrast head computed tomography scan, revealed hypodensity at the border region between the territories of the left anterior cerebral artery and middle cerebral artery. Cerebral digital subtraction angiography revealed the characteristic “puff of smoke” appearance in the right internal carotid artery (ICA), along with severe stenosis of the left ICA. The primary clinical manifestations of MMD include ischemic events such as TIAs, ischemic strokes, and seizures. Recurrent strokes in young patients warrant prompt evaluation for underlying vascular disorders, including MMD. Hemodynamic compromise, a key contributor to recurrent ischemia in this condition, underscores the importance of optimizing collateral circulation to mitigate future stroke risk. Managing hemodynamic stroke in MMD involves adopting a healthy lifestyle and carefully regulating blood pressure to prevent inadequate blood flow and reduce the risk of recurrent strokes.
Keywords
Hemodynamic ischemic stroke
Intracranial stenosis
Moyamoya disease
Recurrent stroke
INTRODUCTION
Moyamoya disease (MMD) is a progressive, occlusive cerebrovascular disorder primarily affecting the circle of Willis and surrounding arteries. The term moyamoya, derived from the Japanese phrase meaning “puff of smoke,” describes the characteristic angiographic appearance of abnormal collateral vessels that develop in response to arterial stenosis.[1] Clinically, patients with MMD predominantly present with ischemic symptoms, observed in approximately 88% of cases, while hemorrhagic manifestations occur in about 11.5%. The risk of recurrent strokes is highest within the first year following diagnosis, reported to be around 18%, subsequently decreasing to approximately 5% annually. MMD is pathologically characterized by spontaneous occlusion of major intracranial arteries and the compensatory formation of fragile collateral vessels at the base of the brain.[2] Impaired collateral function in MMD can predispose to ischemic conditions, particularly during episodes of cerebral hemodynamic instability, thereby triggering hemodynamic stroke.
CASE REPORT
A 34-year-old woman presented with four recurrent episodes of right-sided hemiparesis over eight months. The weakness, predominantly affecting her right upper extremity, impaired grip strength and resolved spontaneously within several days. Her medical history included treated hypertension (mean systolic blood pressure: 140 mmHg), managed with amlodipine 5 mg and clopidogrel 75 mg initiated after her first stroke-like episode. She denied seizures, trauma, infections, or neoplasms. There was no similar family history. She also had a sedentary lifestyle. There was no history of smoking and alcohol consumption. During her most recent hospitalization, vital signs were stable: Blood pressure 120/80 mmHg, pulse 82 bpm, respiratory rate 18 breaths/ min, and afebrile temperature. Physical examination revealed a nutritional status of overweight with body mass index 31.21 kg/m2. There was no increase in jugular venous pressure and no bruit in bilateral temporalis and carotid arteries.. Neurologically, she was fully alert and oriented with intact language function. Cranial nerve assessment demonstrated right-sided central facial palsy and mild lingual nerve palsy. Motor examination confirmed persistent right-sided hemiparesis (4/5 strength) across three admissions, though strength normalized (5/5) in all extremities by discharge.
Laboratory evaluation demonstrated elevated low-density lipoprotein cholesterol and hypertriglyceridemia, while hemoglobin A1C and glucose levels were within normal limits. A head magnetic resonance imaging (MRI) evaluation was performed and showed no signs of cerebral infarction on diffusion-weighted imaging [Figures 1a and b] and T2 fluid-attenuated inversion recovery [Figures 1c and d] in the cortical area. Meanwhile, magnetic resonance angiography (MRA) revealed occlusion of the right middle cerebral artery (MCA) (M1) segment [Figure 1e and f]. It is suggested to be a hemodynamic stroke. Digital subtraction angiography of the right internal carotid artery (ICA) revealed complete right M1 segment occlusion, stenosis of the anterior cerebral artery (ACA) terminus, and compensatory collateral vessel formation within the right MCA territory, displaying the pathognomonic moyamoya (“puff of smoke”) vasculature indicative of Suzuki stage III disease [Figure 2a and b]. Posterior circulation imaging demonstrated analogous collateral networks in the right pericallosal region [Figure 2a]. The left ICA exhibited total occlusion of the left ACA and severe stenosis of the posterior cerebral artery, with partial perfusion maintained through right-sided leptomeningeal collaterals [Figure 2c]. A non-contrast head computed tomography scan during her fourth clinical episode revealed a hypodensity in the left ACA-MCA border (watershed) zone, consistent with hemodynamic infarction [Figure 3a-c].
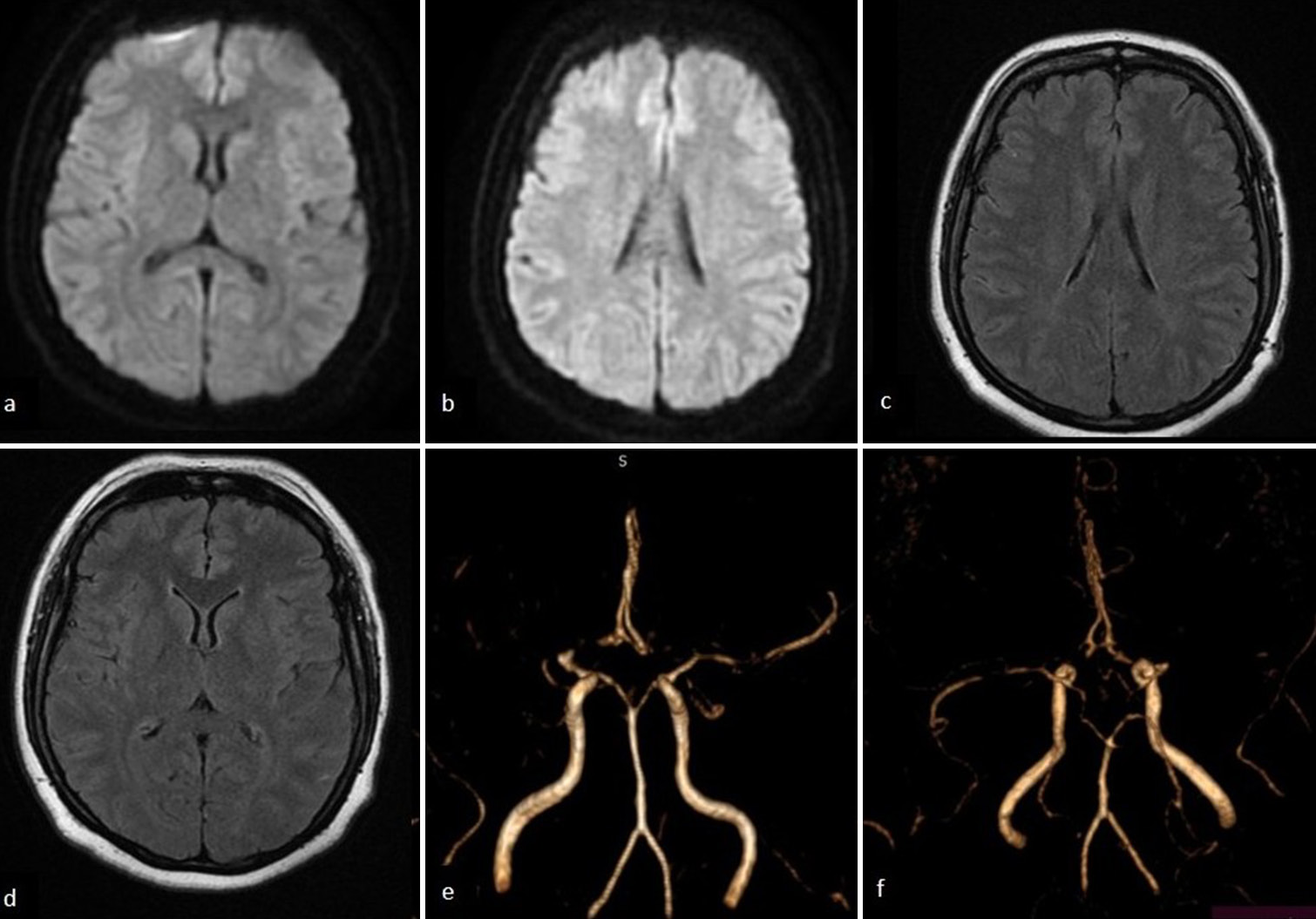
- Magnetic resonance imaging of the head showing no signs of acute infarction on both diffusion-weighted imaging and T2 fluid-attenuated inversion recovery sequences at the level of the (a and c) basal ganglia and (b and d) corona radiata. (e and f) Magnetic resonance angiography revealed an occlusion of the right middle cerebral artery (M1 segment).
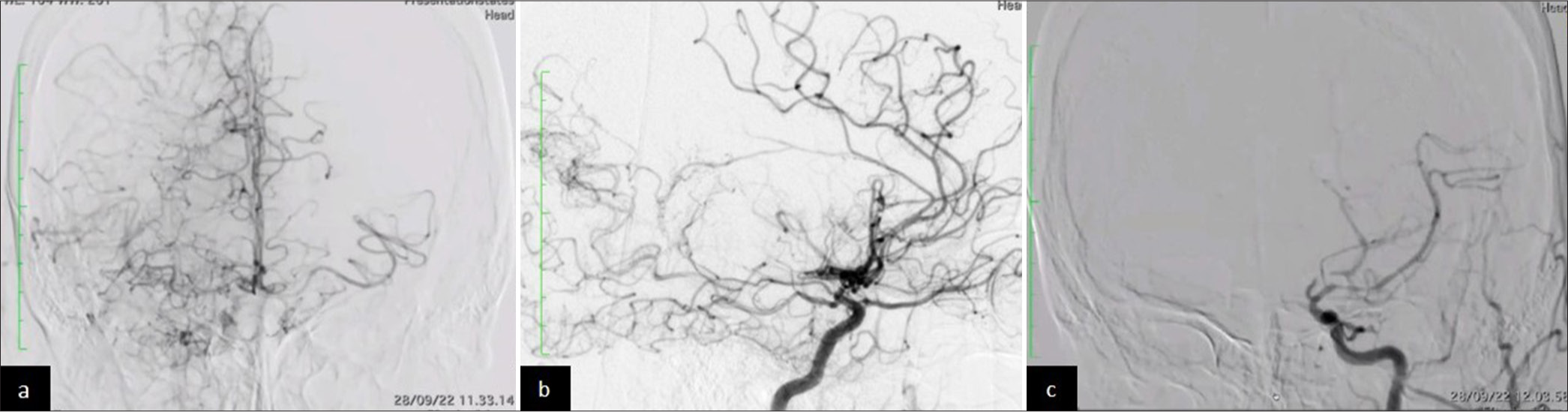
- (a) Right internal carotid artery (ICA) in digital subtraction angiography (DSA) (anteroposterior view): “Puff of smoke” collaterals in the right middle cerebral artery (MCA) lenticulostriate arteries and pericallosal region. (b) Right ICA DSA (lateral view): Prominent collateral vessels reconstituting the right MCA territory. (c) Left ICA DSA (lateral view): Complete anterior cerebral artery occlusion and severe posterior cerebral artery stenosis.
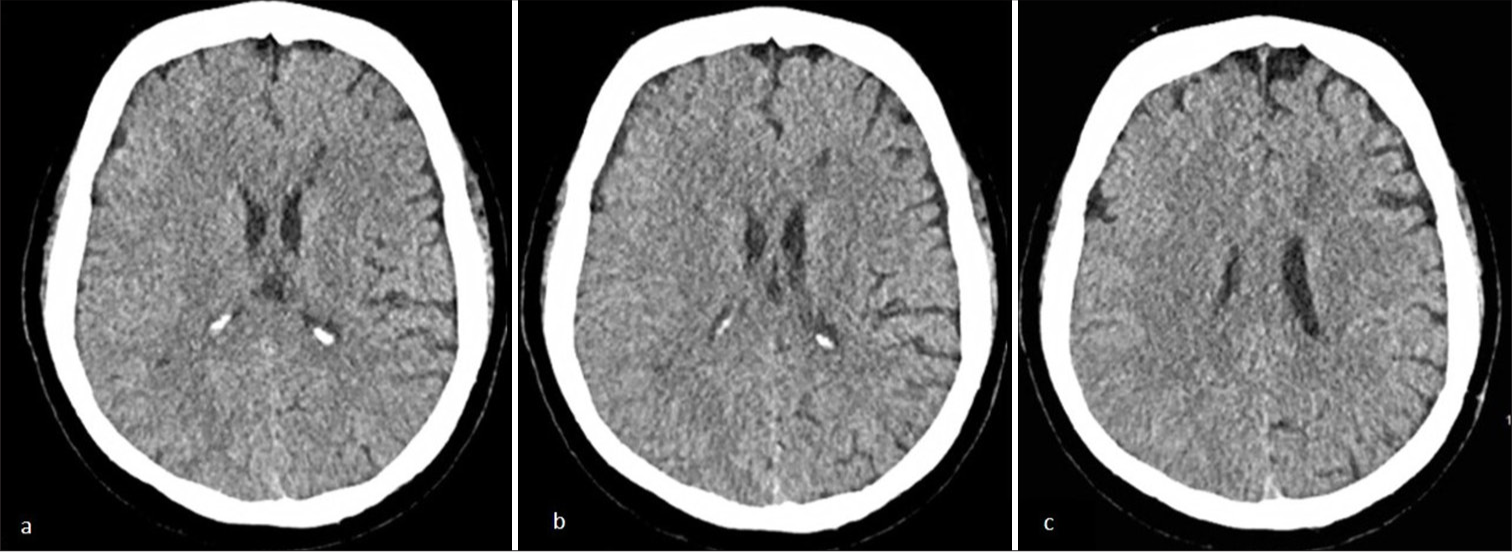
- (a-b) Axial non-contrast head computed tomography scan reveals a hypodense region in the left anterior cerebral artery and middle cerebral artery border (watershed) zone at the level of basal ganglia. (c) The non-contrast head computed tomography scan reveals hypodense region at the corona radiata level..
The patient received conservative therapy, including antihypertensive therapy (amlodipine 10 mg once time daily), anti-dyslipidemia (atorvastatin 20 once time daily), and antiplatelet (clopidogrel 75 mg once time daily).
DISCUSSION
MMD is a progressive, cerebrovascular disorder characterized by spontaneous occlusion, most commonly affecting the ICA and proximal MCA and ACA. This occlusion leads to the formation of delicate collateral vessels at the base of the brain.[2] MMD is most frequently reported in East Asian populations, with a female predominance. Patient ages span from 6 months to 67 years, with a peak incidence in the first decade of life.[1]
The secondary pathological processes in MMD involve the development of collateral vessels in response to chronic occlusion of the major intracranial arteries. These collateral vessels demonstrate a fragmented internal elastic lamina, medial fibrosis, and microaneurysms.[2] Clinically, patients with MMD often experience ischemic events, such as stroke or transient ischemic attacks, in approximately 88% of cases, while hemorrhagic events occur less frequently, in about 11.5% of cases. The risk of recurrent stroke is highest during the first year following diagnosis, reported to be around 18%, and subsequently decreases to approximately 5% annually.[2]
Diagnostic criteria for MMD include: (1) Angiographic evidence of stenosis or occlusion of the terminal ICA and/ or proximal ACA and/or MCA, accompanied by abnormal vascular networks surrounding the occlusive or stenotic lesions. (2) MRI and MRA demonstrating stenosis or occlusion in the same areas, with abnormal vascular networks in the basal ganglia and bilateral flow voids. (3) Exclusion of other potential etiologies. Staging of MMD is performed using the Suzuki grading system, which encompasses Grades I-VI, based on angiographic findings of stenosis, intensification of moyamoya vessels, and increased collateral vessel formation from the external carotid artery. In this patient, the findings are consistent with Suzuki Stage III, characterized by intensification of moyamoya vessels and increased stenosis or occlusion of the MCA and ACA.[2]
Management of MMD involves both medical and surgical approaches.[2] While no specific pharmacologic regimen has demonstrated definitive efficacy in treating MMD, antiplatelet therapy is commonly employed to prevent recurrent strokes.[2] Surgical intervention is indicated for patients with recurrent cerebral ischemic symptoms, evidence of reduced cerebral blood flow on imaging or metabolic studies, and a Suzuki stage of IV or higher.[2] Surgical management encompasses two techniques: Direct and indirect revascularization. The direct technique, although more technically challenging, typically provides faster revascularization, as seen in the superficial temporal artery (STA) to MCA bypass, which uses the STA as the main blood supply. Indirect revascularization techniques, while technically less demanding, generally take longer to achieve adequate revascularization.[2]
Hemodynamic stroke refers to ischemic stroke resulting from cerebral hypoperfusion rather than embolism or localized vascular pathology.[3] Contributing factors to hemodynamic stroke include ICA stenosis and occlusion, as observed in MMD, as well as vertebral artery obstruction and systemic hypotension.[4] Diagnosis of hemodynamic stroke relies on the detection of infarction in the border zone (watershed areas) between major cerebral arteries territories.[3] The vulnerability of watershed areas is attributed to their distal field location, where perfusion pressure is lowest, and repeated episodes of hypotension can predispose to watershed infarcts.[5] Collateral blood flow also plays a critical role in cerebral hemodynamic status, with poor collateral flow being a hallmark of impaired cerebral hemodynamics.[2]
In this patient, episodes of relative hypotension, combined with poor collateral flow, likely contributed to hemodynamic stroke, as evidenced by recurrent right-sided hemiparesis resolving after more than 24 h. Neuroimaging findings, specifically hypodense areas in the watershed zone, were consistent with hemodynamic stroke. Preventive management for hemodynamic stroke includes antiplatelet therapy, lipid reduction, smoking cessation, and increased physical activity.[6] Blood pressure management remains a subject of debate; however, careful control is recommended to avoid excessively low blood pressure that may precipitate hypoperfusion. Recent studies, including those involving stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) patients with hemodynamic ischemic stroke patterns, suggest no increased risk of stroke with systolic blood pressure below 140 mmHg.[6] Surgical management may be considered in this patient due to recurrent ischemic events.
CONCLUSION
MMD is a progressive, cerebrovascular disorder characterized by spontaneous occlusion and impaired collateral function, which contribute to recurrent hemodynamic ischemic stroke. Hemodynamic stroke management in MMD involves maintaining a healthy lifestyle and carefully controlling blood pressure to prevent hypoperfusion and recurrent hemodynamic stroke.
Ethical approval:
Institutional Review Board approval is not required.
Declaration of patient consent:
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest:
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation:
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Epidemiology, diagnosis and treatment of moyamoya disease (Review) Exp Ther Med. 2019;17:1977-84.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial cerebrovascular occlusive disease In: Harrigan MR, Deveikis JP, eds. Handbook of cerebrovascular disease and neurointerventional technique (3rd ed). Germany: Springer Nature; 2018. p. :1025-39.
- [CrossRef] [Google Scholar]
- Haemodynamic stroke: Clinical features, prognosis, and management. Lancet Neurol. 2010;9:1008-17.
- [CrossRef] [PubMed] [Google Scholar]
- Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009;360:1226-37.
- [CrossRef] [PubMed] [Google Scholar]
- The pathophysiology of watershed infarction in internal carotid artery disease: Review of cerebral perfusion studies. Stroke. 2005;36:567-77.
- [CrossRef] [PubMed] [Google Scholar]
- Hemodynamic stroke: Emerging concepts, risk estimation, and treatment. Stroke. 2024;55:1940-50.
- [CrossRef] [PubMed] [Google Scholar]







