Translate this page into:
Quality of Sleep and Sleep Disorders in Patients with Parkinsonism: A Polysomnography Based Study from Rural South India
Address for correspondence: Dr. Dushyanth Babu Jasti, Department of Neurology, SVIMS, Tirupati - 517 507, Andhra Pradesh, India. E-mail: dushyanthjasti@yahoo.co.in
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective:
The objective of this study is to study the quality of sleep, sleep disorders, and polysomnographic profile in Parkinsonism patients from rural areas and to correlate polysomnographic profile with the staging of disease and with sleep questionnaire.
Materials and Methods:
Between May 2014 and December 2015, 168 Parkinsonism patients were prospectively screened using sleep questionnaire; Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Parkinson Disease Sleep Score-2 (PDSS-2). Sixty patients underwent overnight polysomnography subsequently.
Results:
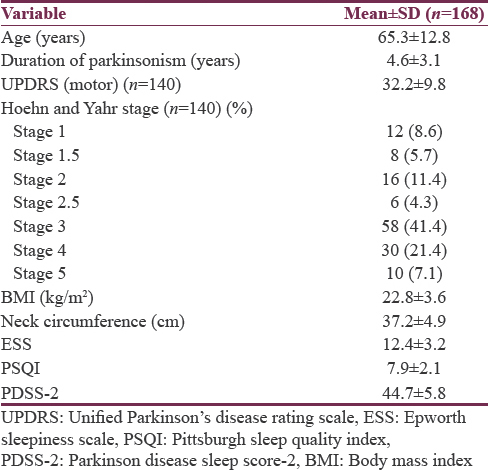
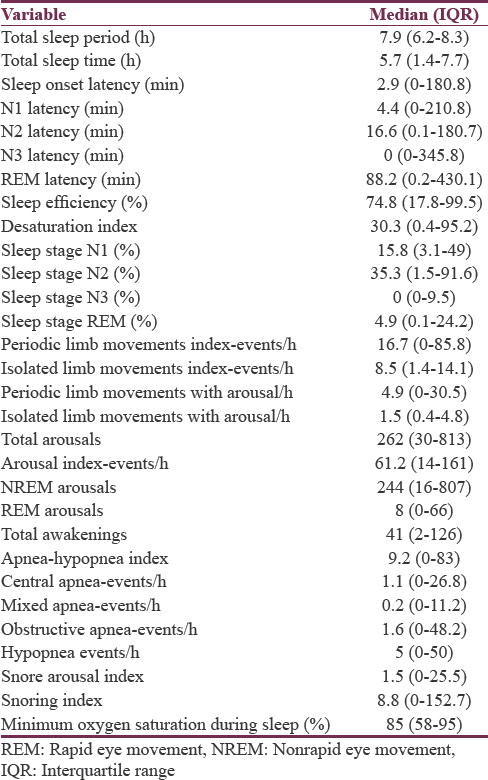
The mean age of 168 patients in the study was 65.3 ± 12.8 years. The mean duration of Parkinsonism was 4.6 ± 3.1 years. The mean ESS, PSQI and PDSS-2 were 12.4 ± 3.2, 7.9 ± 2.1 and 44.7 ± 5.8, respectively. A total of 148 patients (88.1%) had poor quality sleep, which was reported only in 37 patients (22%). Excessive daytime sleepiness (80%) and insomnia (76.7%) were most common symptoms. Polysomnographic profile showed poor sleep efficiency (median interquartile range [IQR] 74.8% [17.8%–99.5%]), reduced slow wave sleep (median [IQR] 0% [0%–9.5%]), and reduced rapid eye movement [REM] sleep (median (IQR) 4.9% [0.1%–24.2%]). Sleep disorders in the study were sleep fragmentation (n = 60, 100%), obstructive sleep apnea syndrome (n = 40, 66.7%), central sleep apnea syndrome (n = 6, 10%), and periodic limb movement disorder (n = 52, 86.7%). Two patients had REM sleep behavioral disorder clinically. There was statistically significant positive correlation between staging of disease, sleep latencies, and sleep questionnaire.
Conclusion:
Sleep is impaired in majority of Parkinsonism patients which needs to be diagnosed early and managed effectively. Patient education and awareness programs in rural areas regarding sleep disorders in Parkinsonism are required for early diagnosis.
Keywords
Parkinsonism
polysomnography
rural areas
sleep disorders
sleep questionnaire
INTRODUCTION
Parkinsonism is an extrapyramidal movement disorder which is characterized by rest tremor, bradykinesia, rigidity, and postural instability secondary to degeneration of dopaminergic neurons in the striatonigral region.[1] Apart from dopaminergic system involvement; cholinergic, noradrenergic, and serotonergic systems[2] are also involved in Parkinsonism which is responsible for nonmotor symptoms. Nonmotor symptoms are most disabling symptoms of Parkinsonism rather than motor symptoms which are often neglected. Sleep disturbances are one of the most common nonmotor symptoms which were first described by James Parkinson.[3] Sleep disturbances are most disabling due to significant impact on the quality of life. Very often, details about sleep are omitted from history by physicians while assessing the patient. Moreover, patients and caregivers do not recognize the importance of sleep, thereby under report sleep disturbances until those disturbances become severe.
Sleep disorders are relatively common in Parkinson's disease with reported incidence ranging approximately 40%–90%.[4567] Nocturnal sleep disturbances frequency in Parkinson's disease ranges between 60% and 90%. Excessive daytime sleepiness is also seen in 50% of Parkinson's disease patients, which is most common in mild-to-moderate grade of patients.[8910] Sleep disorders need to be identified early because early detection and better management cause improvement in motor symptoms also.
Central sleep regulation centers degeneration in the thalamus and brainstem are implicated in sleep disturbances associated with Parkinsonism. Degeneration of raphe nucleus and locus coeruleus in Parkinsonism results in basic rapid eye movement (REM) and nonrapid eye movement (NREM) sleep architecture disruption. Dopaminergic dysfunction and degeneration of neurons with destabilization of switch and regulators result in rapid sleep transition.
Sleep disturbances seen in Parkinsonism include sleep fragmentation, sleep apnea, restless leg syndrome, REM sleep behavior disorder (RBD), periodic limb movement disorder (PLMD), and circadian rhythm disorders. These disorders usually increase as disease advances. Sleep fragmentation is very common in Parkinson's disease which is characterized by frequent awakenings during sleep.[11] A study conducted in the United Kingdom showed 76% of 220 Parkinsonism patients had disrupted night sleep. Sleep fragmentation is mainly due to inability to roll over in bed, foot dystonia, frequent urination, and tremor.[4] Sleep apnea is seen in approximately 20%–40% of Parkinsonism patients who are referred for polysomnography to a sleep laboratory.[12131415] Obstructive sleep apnea is relatively more common in patients with Parkinsonism than other types of sleep apnea. Parkinsonian patients who are excessively drowsy during daytime without any specific cause or those with a positive history for sleep apnea need to be thoroughly evaluated with overnight polysomnography. In patients with multiple system atrophy (MSA), nocturnal apneic episodes and episodes of stridor with glottis closure are seen,[1617] who respond poorly to continuous positive airway pressure. Periodic limb movements, especially during NREM sleep are more common in Parkinson's disease patients.[18] Depression is one of the major cause of sleep fragmentation and delayed onset of sleep in Parkinson's disease patients. Depression is seen in approximately 40% of patients with Parkinson's disease.[19] REM sleep behavior disorder is seen in 15%–32% of Parkinson's disease patients[20] when evaluated based on clinical criteria. But when evaluated by polysomnography, 33% of Parkinson's disease patients had REM sleep behavior disorder and 58% showed muscle atonia loss in REM sleep.[21] REM sleep behavior disorder is more common in dementia with Lewy bodies (DLB) and MSA.[222324] Excess daytime somnolence is seen in 15.5% of Parkinson's disease patients.[25] Excess daytime somnolence is associated with advanced Parkinsonism, cognitive dysfunction, frequent hallucinations, greater disability, and longer duration of levodopa therapy.
Data regarding polysomnography and sleep scales based evaluation of sleep in Parkinsonism patients from rural population of India are sparse. Hence, we conducted a study to assess the pattern of sleep, quality of sleep, and type of sleep disorders in Parkinsonism patients from rural areas of Andhra Pradesh.
MATERIALS AND METHODS
During the period May 2014 to December 2015, consecutive patients diagnosed to have and treated for Parkinsonism (which includes idiopathic Parkinson's disease based on the United Kingdom Parkinson's Disease Society Brain Bank criteria, Parkinson-plus syndromes, and secondary Parkinsonism) at Sri Venkateswara Institute of Medical Sciences, Tirupati, were included in the study. All the patients included in the study belonged to rural areas of Rayalaseema region, Andhra Pradesh, as per Indian government urban–rural classification. Patients with Parkinsonism from urban and semi-urban areas were excluded from the study. This study was approved by the institutional ethics committee, and written informed consent was obtained from the patients for their participation in the study.
Study procedure
This study was carried out in two stages.
First stage of the study
In the first stage, 168 consecutive patients with Parkinsonism who had given consent to participate in the study were screened for excessive daytime sleepiness by Epworth Sleepiness Scale (ESS).[26] The same patients were also screened for poor sleep quality by Pittsburgh Sleep Quality Index (PSQI)[27] and Parkinson's Disease Sleep Score-2 (PDSS-2).[28] Excessive daytime sleepiness was defined as an ESS score >10. Poor sleep quality was defined as PSQI score more than 5 or PDSS-2 score more than 50. Of the screened Parkinsonism patients, in whom ESS was 10 or greater/PSQI was 5 or more/PDSS-2 was 50 or more, 70 patients who gave consent for second stage of the study were selected for undergoing detailed evaluation and polysomnography. Of the 70 patients, 10 patients were not cooperative for overnight polysomnography.
Second stage of the study
These sixty study participants underwent a detailed evaluation that included clinical history focused on sleep-related symptoms, associated conditions, and comorbidities. Idiopathic Parkinson's disease patients were graded as per Hoehn and Yahr staging. All participants were subjected to a detailed ear, nose, and throat examination to rule out significant upper airway obstruction.
Anthropometric measurements
Body-mass index was calculated as body weight (in kg)/height (in m).[2] Neck circumference (in cm) was measured at the level of cricothyroid membrane. Neck length (NL, cm) was measured from the occipital tubercle to the C7 vertebra prominence. H (height) to NL ratio was calculated by dividing the H (cm) by NL (cm). Waist circumference (cm) was measured midway between the lower rib margin and the anterior superior iliac spine. Hip circumference (in cm) was measured at the maximum circumference of the buttocks while the participant was standing with feet placed together. Mean of three readings of each circumference was taken for the calculation of waist–hip ratio. Mid-arm circumference was measured using a nonstretchable tape, midway between the acromion process of the scapula and the olecranon process.
Overnight polysomnography recording
Patients with Parkinsonism were called for sleep study to the sleep laboratory at 8 pm, on the day of their appointment and were hooked to Sleep scan Vision (Bio-logic Systems Corp, Illinois, USA). Various parameters that were monitored included electroencephalography (EEG), electrooculography, electrocardiography, chin and leg electromyography, nasal airflow, tracheal breath sounds, thoracic wall and abdominal movements, transcutaneous oxygen saturation, body position, and continuous positive airway pressure titration when required. Total sleep period, total sleep time, sleep latency, REM sleep latency, sleep efficiency, sleep stages, wake after sleep onset, sleep cycles, stage shifts, and arousal index were the parameters which were scored initially while analyzing a sleep record. Apnea was defined as cessation of oronasal airflow for >10 s. Obstructive sleep apnea was scored when airflow is absent but respiratory efforts were present. Hypopnea was defined as a discernible reduction in respiratory airflow of 50%–90% for >10 s accompanied by a decrease of 4% or more in oxyhemoglobin saturation during sleep. Apnea-hypopnea index (AHI) was calculated using the formula – AHI = (total number of obstructive apneas + total number of hypopneas)/total sleep time (h). Sleep-disordered breathing is classified into mild if 5–15 events, moderate if 15.1–30 events and severe if more than 30 events. Based on oxygen saturation, sleep-disordered breathing is classified as mild –85%–89%, moderate –80%–84%, and severe –79% or less. Arousal is defined as either a transient return of alpha or beta activities or a change from delta or theta activities in EEG lasting from 3 to 14 s. Arousal index is defined as number of arousals per hour of sleep and scored as normal up to 5. Sleep fragmentation is defined as microarousals lasting for more than 3 s and < 10 s with arousal index more than 9.9/hour. Periodic limb movement index is defined as number of periodic limb movements per hour of sleep. It is classified as mild if 5–25, moderate if 25.1–50 and severe if more than 50 events.
Statistical analysis
Data were recorded on a predesigned pro forma and managed using Microsoft Excel (Microsoft Corp, Redmond, WA). All the entries were double-checked for any possible error. Descriptive statistics for the categorical variables were performed by computing the frequencies (percentages) in each category. For the quantitative variables, approximate normality of the distribution was assessed. Variables following normal distribution were summarized by mean and standard deviation. Variables not following a normal distribution were summarized as median (interquartile range [IQR]). The association between ESS, PSQI, PDSS scales, and the Hoehn and Yahr staging was studied using Chi-square test. Correlation between ESS, PSQI, PDSS scales, staging and duration of Parkinsonism with polysomnographic parameters was studied using Pearson's correlation coefficient. Among patients with Parkinsonism who underwent polysomnography, demographic, anthropometric, and laboratory variables were compared between patients with and without sleep disorders with two-sample t-test, Mann–Whitney U-test as appropriate. Statistical softwares PASW Statistics 18, Release 18.0.0, (IBM SPSS Statistics, Somers NY, USA); Systat 12, Version 12.00.08 (Systat Software, Inc, Chicago IL, USA); and Med Calc Version 11.3.0 for Windows 2000/XP/Vista/7 (MedCalc Software bvba, Belgium) were used for statistical analysis.
RESULTS
First stage of the study
A total of 168 consecutive patients with Parkinsonism who consented to participate in the study were subsequently screened for excessive daytime somnolence by ESS. Quality of sleep was assessed in these patients using PSQI and PDSS-2. Patients who had ESS >10/PSQI >5/PDSS-2 >50 were included in second part of the study. The study plan is shown in Figure 1.
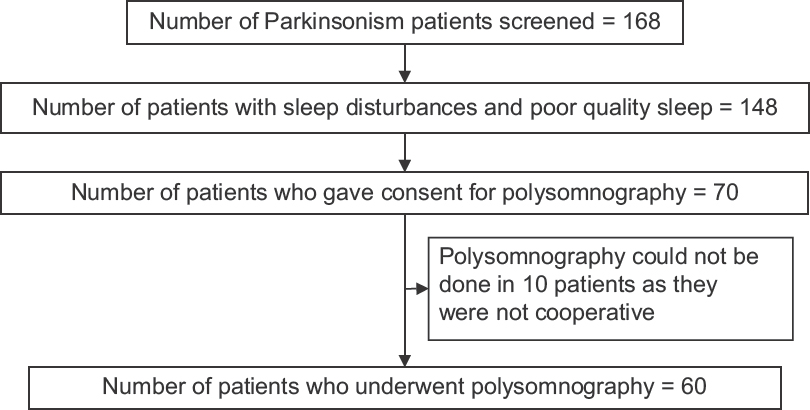
- Study plan
The mean age of the patients who participated in first part of the study (n = 168) was 65.3 ± 12.8 years (mean ± standard deviation); 128 (76.2%) patients were male and 40 (23.8%) were female. A total of 140 (83.3%) patients had idiopathic Parkinson's disease, whereas 24 (14.3%) patients had Parkinson-plus syndrome. Four (2.4%) patients had secondary Parkinsonism, all were drug induced. Phenotypic characteristics of 168 patients who participated in first part of the study are shown in Table 1.
Sleep disturbances and poor quality sleep as assessed by ESS/PSQI/PDSS-2 were present in 148 patients (88.1%). However, only 37 patients (22%) reported symptoms of sleep disturbance voluntarily.
Second stage of the study
Out of 148 patients who were diagnosed to have sleep disorders, 70 patients gave consent to participate in overnight polysomnography. In 10 patients, polysomnography could not be done as patients were not cooperative. A total of 60 patients underwent overnight polysomnography as second part of the study. Phenotypic characteristics of 60 Parkinsonism patients who underwent polysomnography are shown in Table 2.

Thirty (50%) patients were obese, 20 (33.3%) patients were overweight, and 10 (16.7%) patients were of normal weight. Out of 60 patients who underwent polysomnography, 54 (90%) patients had idiopathic Parkinson's disease, whereas 6 (10%) patients had Parkinson-plus syndrome. Of the 6 patients with Parkinson-plus syndrome, two had progressive supranuclear palsy, two had corticobasal degeneration, and two had multiple system atrophy-Parkinsonian type.
Most common symptoms of sleep disturbance in the present study were excessive daytime sleepiness (80%), followed by insomnia (76.7%), rigidity (46.7%), snoring (30%), cramps (30%), nocturia (16.7%), limb movements (13.3%), and vivid dreams (3.3%). Out of 60 Parkinsonism patients who underwent polysomnography, all patients were treated with levodopa. Along with levodopa, 48 patients were treated with dopamine agonists, 26 patients were treated with amantadine and 4 patients were treated with catechol-O-methyl transferase inhibitors.
Polysomnographic parameters
Polysomnography showed reduced sleep efficiency; reduced quality, quantity of slow wave, and REM sleep. Details of polysomnography are shown in Table 3.
Sleep disorders in Parkinsonism demonstrated by polysomnography in the present study were sleep fragmentation (n = 60, 100%), obstructive sleep apnea syndrome (OSAS, n = 40, 66.7%), central sleep apnea syndrome (n = 6, 10%), and PLMD (n = 52, 86.7%).
AHI of 5 or more was observed in 40 (66.7%) patients and all of them had ESS >10. Median (IQR) AHI was 9.2 (0–83). Twelve (20%) patients had mild OSAS, 8 (13.3%) patients had moderate OSAS whereas 20 (33.3%) patients had severe OSAS. Comparing data of patients with OSAS and patients without OSAS showed the presence of rigidity (P = 0.007), isolated leg movements index per hour (P = 0.033), and isolated leg movements with arousal (P = 0.012) are statistically significant factors for OSAS.
Sleep fragmentation with arousal index >9.9/h is seen in all patients (100%). Periodic limb movement index of 5 or more was observed in 52 (86.7%) patients who underwent polysomnography. Twenty-eight (46.7%) patients had mild PLMD, 14 (23.3%) patients had moderate PLMD while 10 (16.7%) patients had severe PLMD. Comparison of patients with PLMD and patients without PLMD did not show any statistically significant demographic, clinical, anthropometric, and polysomnographic variables. Two patients had RBD clinically but none of the patients had evidence of RBD on polysomnography.
A statistically significant positive correlation was observed between ESS and sleep-onset latency (P = 0.001); N1 latency (P = 0.003); and N2 latency (P = 0.0001). A statistically significant negative correlation was observed between ESS and sleep efficiency (P = 0.0001); N1 stage duration (P = 0.041); N2 stage duration (P = 0.0001).
A statistically significant positive correlation was observed between PSQI and sleep-onset latency (P = 0.004); N1 latency (P = 0.014); N2 latency (P = 0.0001); REM latency (P = 0.034); and total awakenings (P = 0.01). A statistically significant negative correlation was observed between PSQI and sleep efficiency (P = 0.0001); N2 stage duration (P = 0.0001); and REM sleep duration (P = 0.055).
A statistically significant positive correlation was observed between PDSS-2 and sleep-onset latency (P = 0.005); N1 latency (P = 0.008); N2 latency (P = 0.0001); REM latency (P = 0.035); and total awakenings (P = 0.021). A statistically significant negative correlation was observed between PDSS-2 and sleep efficiency (P = 0.0001); N2 stage duration (P = 0.0001); and REM sleep duration (P = 0.055).
A statistically significant positive correlation was observed between Hoehn and Yahr staging of disease and ESS (P = 0.0001); PSQI (P = 0.0001); PDSS-2 (P = 0.0001); periodic limb movement index (P = 0.023); periodic limb movements with arousal (P = 0.012); snore arousal (P = 0.006); and snoring index (P = 0.003). A statistically significant negative correlation was observed between staging of disease and sleep efficiency (P = 0.002); N2 stage duration (P = 0.021).
A statistically significant positive correlation was observed between duration of disease and snore arousal (P = 0.004); snoring index (P = 0.003).
DISCUSSION
Sleep symptoms are often neglected in Parkinsonism patients from rural areas, where there is low level of knowledge and awareness. The present study is undertaken to assess burden and type of sleep disturbances in Parkinsonism patients from rural areas of Andhra Pradesh. Sleep disturbances were present in 88.1% of patients in the present study when assessed by various sleep questionnaires. However, only 37 patients (22%) reported symptoms of sleep disturbance voluntarily. This depicts the level of knowledge regarding the importance of sleep symptoms and sleep disorders in the rural population. In a study conducted by Norlinah et al.,[29] prevalence of sleep disorders was 81% which was 78.3% according to a study conducted by Alatriste-Booth et al.[30] According to Ferreira et al.[31] from Chandigarh, sleep disturbances were reported in 98% of drug-naïve patients with Parkinson's disease. Sleep disturbances were reported in 70% of patients as per Se lvaraj and Keshavamurthy[32] from Chennai. As per Kumar et al.[6] from Delhi, sleep disorders were seen in 42% of patients with Parkinson's disease. However, published studies were not confined to the rural population. Indian studies addressing sleep disorders in Parkinsonism patients from rural areas are very sparse.
Polysomnographic parameters in Parkinsonism
In patients with Parkinsonism, sleep architecture is altered even before manifestation of motor symptoms. Disturbances in polysomnography parameters associated with Parkinsonism are poor sleep efficiency, sleep fragmentation, and decrease in quality and quantity of N3 and REM sleep as per literature. In the present study also, N3 sleep and REM sleep are significantly low with poor sleep efficiency. Reduction in N3 sleep could be part of both advanced age and Parkinsonism. Comparison of polysomnographic parameters with other published studies[29303334] is shown in Table 4.
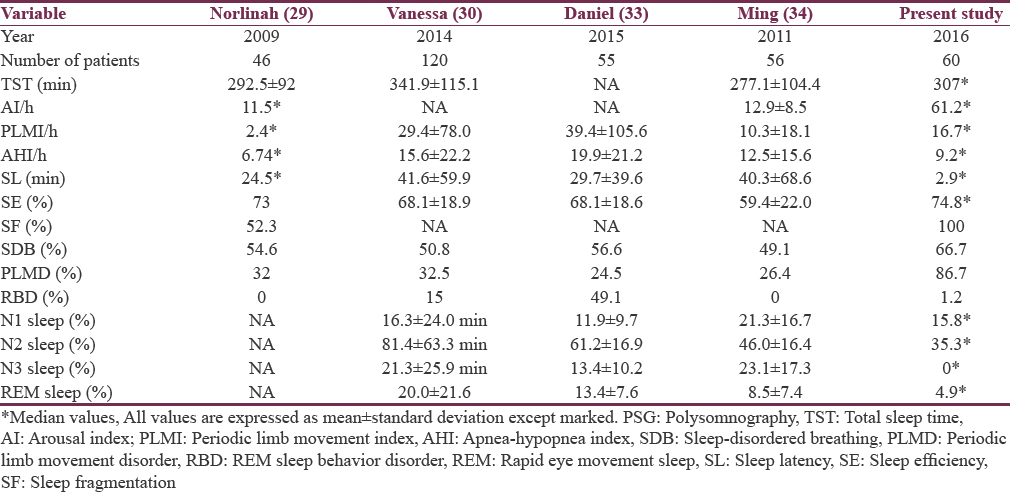
According to Joy et al.[35] from Bengaluru, in drug-naïve Parkinson's disease patients, the mean sleep efficiency was 68.3% ± 21.3% which was comparable to the present study. The mean N1 sleep % was 14.4% ± 7.6%, mean N2 sleep % was 36.5% ± 13.1%, mean N3 sleep % was 4.9 ± 4.9%, and mean REM sleep % was 13.4 ± 4.1% according to Joy et al.[35] These results were comparable to the present study which shows poor sleep efficiency and reduced N3 sleep quality and quantity. Polysomnographic parameters in the present study were more or less similar to other studies from urban population.
Most common sleep disorders in Parkinsonism patients who underwent polysomnography were sleep fragmentation (n = 60, 100%) followed by PLMD (n = 52, 86.67%) and obstructive sleep apnea syndrome (n = 40, 66.67%). There are very few Indian studies which studied the prevalence of sleep disorders in Parkinsonism by polysomnography. However, polysomnography-based studies from rural population are very sparse from Indian literature.
Sleep disordered breathing in Parkinsonism
Sleep-disordered breathing was seen in 73% of patients in the present study. Obstructive sleep apnea syndrome (66.7%) was more common than central sleep apnea (10%). Mild OSAS was seen in 12 patients (20%), moderate OSAS was seen in 8 patients (13.3%), and severe OSAS was seen in 20 patients (33.3%). Comparison of OSAS in parkinsonism with other published studies[29303336] is shown in Table 5.
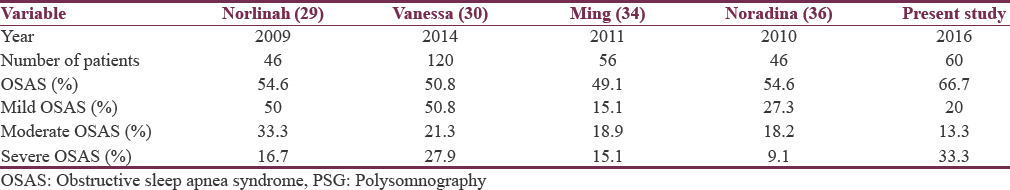
Severe obstructive sleep apnea syndrome was relatively more in the present study when compared to other published studies probably due to more number of obese patients included in the present study. In the present study, 36 (60%) enrolled patients were obese which might be the reason for more number of severe OSAS cases.
Periodic limb movement disorder in Parkinsonism
PLMD is seen in Parkinsonism patients as the disease advances in a greater proportion. In the present study, PLMD was seen in 52 (86.67%) enrolled patients. According to Norlinah et al.[29] and Alatriste-Booth et al.,[30] mild PLMD was seen in 42.8% and 58.9%, respectively; moderate PLMD was seen in 28.6% and 15.4%, respectively, severe PLMD was seen in 28.6% and 25.7%, respectively, which were almost similar to our study.
Sleep fragmentation in Parkinsonism
Sleep fragmentation was present in all patients as per polysomnography with high arousal index, more frequent arousals, and awakenings. According to various other published studies, sleep fragmentation was present in at least 50% of cases.[29] As this study was done in two steps, patients with sleep disorders could be screened effectively using various sleep questionnaires during first part of the study. Hence, patients who were enrolled in the second part of the study had some or other sleep disorder which accounted for more cases of sleep fragmentation when compared with other published studies.
Rapid eye movement sleep behavior disorder in Parkinsonism
REM sleep behavior disorder was seen in two patients based on history but was not documented based on polysomnography. RBD was not documented in the present study unlike other studies where it ranged between 3% and 50%. This was probably due to less number of Parkinson plus patients who underwent polysomnography. REM sleep behavior disorder is more common as the disease advances.
Pattern of sleep disorders in Parkinsonism patients was comparable to other published national and international studies which were done predominantly in urban population.
Limitations of the present study
-
This study is a hospital-based study, so it is not accurate to measure of community-based prevalence of sleep disorders in Parkinsonism
-
As normal polysomnography values are not available from large Indian studies, data in the present study are not comparable.
CONCLUSION
Our study highlights poor level of knowledge regarding sleep disorders and sleep symptoms in rural population. Sleep is impaired in majority of Parkinsonism patients which needs to be diagnosed early and managed effectively. Patient education and awareness programs in rural areas regarding sleep disorders in Parkinsonism are required for early diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- Parkinson's disease: Clinical features and diagnosis. J NeurolNeurosurg Psychiatry. 2008;79:368-76.
- [Google Scholar]
- Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991;14:153-97.
- [Google Scholar]
- Sleep disturbances associated with Parkinson's disease. Parkinsons Dis 2011 2011:219056.
- [Google Scholar]
- A community-based study of sleep disorders in patients with Parkinson's disease. MovDisord. 1998;13:895-9.
- [Google Scholar]
- Evaluation of somnolence in Parkinson's disease: Comparison with age- and sex-matched controls. Neurology. 2002;58:465-8.
- [Google Scholar]
- Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: A survey by the Canadian movement disorders group. JAMA. 2002;287:455-63.
- [Google Scholar]
- Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology. 2001;57:1392-6.
- [Google Scholar]
- Sleep and periodic leg movement patterns in drug-free patients with Parkinson's disease and multiple system atrophy. Sleep. 2000;23:361-7.
- [Google Scholar]
- Sleep breathing disorders in patients with idiopathic Parkinson's disease. Respir Med. 2003;97:1151-7.
- [Google Scholar]
- Parkinson's disease and sleepiness: An integral part of PD. Neurology. 2002;58:1019-24.
- [Google Scholar]
- Sleep disorders in multiple system atrophy: A correlative video-polysomnographic study. Sleep Med. 2004;5:21-30.
- [Google Scholar]
- Abnormal respiration and sudden death during sleep in multiple system atrophy with autonomic failure. Neurology. 1990;40:677-9.
- [Google Scholar]
- Movement disorders in sleep: Parkinson's disease and restless legs syndrome. Biomed Tech (Berl). 2003;48:62-7.
- [Google Scholar]
- Health-related quality of life and sleep disorders in Parkinson's disease. NeurolSci. 2003;24:209-10.
- [Google Scholar]
- Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson's disease. Neurology. 1998;51:526-9.
- [Google Scholar]
- Delayed emergence of a Parkinsoniandisorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology. 1996;46:388-93.
- [Google Scholar]
- REM sleep behavior disorders in multiple system atrophy. Neurology. 1997;48:1094-7.
- [Google Scholar]
- Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology. 2003;61:40-5.
- [Google Scholar]
- Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology. 2004;62:401-6.
- [Google Scholar]
- Daytime sleepiness and the COMT val158met polymorphism in patients with Parkinson disease. Sleep. 2006;29:108-11.
- [Google Scholar]
- A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540-5.
- [Google Scholar]
- The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213.
- [Google Scholar]
- Parkinson's disease sleep scale – Validation of the revised version PDSS-2. MovDisord. 2011;26:644-52.
- [Google Scholar]
- Sleep disturbances in Malaysian patients with Parkinson's disease using polysomnography and PDSS. Parkinsonism RelatDisord. 2009;15:670-4.
- [Google Scholar]
- Prevalence and correlates of sleep disorders in Parkinson's disease: Apolysomnographic study. Arq Neuropsiquiatr. 2015;73:241-5.
- [Google Scholar]
- Sleep disturbances in drug naïve Parkinson's disease (PD) patients and effect of levodopa on sleep. Ann Indian Acad Neurol. 2014;17:416-9.
- [Google Scholar]
- A polysomnographic study of Parkinson's disease sleep architecture. Parkinsons Dis. 2015;2015:570375.
- [Google Scholar]
- Case control polysomnographic studies in Parkinson's disease. Plos one. 2011;6:1-7.
- [Google Scholar]
- Alterations in polysomnographic (PSG) profile in drug-naïve Parkinson's disease. Ann Indian AcadNeurol. 2014;17:287-91.
- [Google Scholar]
- Sleep-disordered breathing in patients with Parkinson's disease. Singapore Med J. 2010;51:60-4.
- [Google Scholar]






