Translate this page into:
Primary central nervous system Hodgkin’s lymphoma – A case report and review of literature
*Corresponding author: Vengalathur Ganesan Ramesh, Department of Neurosurgery, Chettinad Hospital and Research Institute, Kelambakkam, Tamil Nadu, India. drvgramesh@hotmail.com
-
Received: ,
Accepted: ,
How to cite this article: Thrinadh B, Karthikeyan KV, Raghavan V, Sathyaseelan M, Ramesh VG. Primary central nervous system Hodgkin’s lymphoma – A case report and review of literature. J Neurosci Rural Pract. 2025;16:109-14. doi: 10.25259/JNRP_402_2024
Abstract
Hodgkin’s lymphoma (HL) is primarily a disease affecting the lymph nodes. Extranodal involvement is rare. Primary central nervous system involvement in HL is extremely rare. The diagnosis is established by morphology and distinct immune-histochemical staining of the biopsies. A 76-year-old male patient presented with speech disturbance and weakness of the right upper and lower limbs. Magnetic resonance imaging of the brain showed an intraparenchymal mass lesion in the left frontal lobe with features of a high-grade tumor. Craniotomy and complete excision of the mass lesion were done. With histopathological examination and immunohistochemistry, the diagnosis of HL was made. Whole-body positron emission tomography did not reveal any other focus. The patient is being followed up with further oncological management. Only 26 cases of primary HL have been reported in the literature. Hence, this case is presented for its rarity. The previously reported cases and the recent concepts in the pathogenesis and treatment have also been reviewed.
Keywords
Central nervous system lymphoma
Hodgkin’s lymphoma of central nervous system
Primary central nervous system Hodgkin’s lymphoma
INTRODUCTION
Hodgkin’s lymphoma (HL) commonly affects the lymph nodes. Central nervous system (CNS) lymphoma can present either as secondary CNS involvement by systemic lymphoma which is more common or rarely as primary CNS lymphoma which is restricted to the brain, leptomeninges, spinal cord, or eyes.[1-4] CNS involvement in HL is shown to occur in <0.2–0.5% of HL patients when compared to 5–30% CNS involvement in NHL patients.[5,6] Primary CNS lymphoma of CNS is NHL and is usually secondary to an immunocompromised state and HL has secondary CNS involvement usually in the leptomeninges. Primary CNS involvement is extremely rare and only about 26 cases have been reported so far. Due to its rarity, the exact pathogenesis, its relation to the immune status of the patient, the exact role of Epstein-Barr Virus, its activation of regulator T (Treg) cells, and the role of programmed cell death ligand (PD-L) 1 in the genesis of primary CNS HL are yet to be investigated fully.
CASE REPORT
A 76-year-old male presented to the Department of Neurosurgery with behavioral change, speech disturbance, with weakness of the right upper and lower limbs for two weeks. The patient was conscious, disoriented, and confused and responded slowly to commands. He had right hemiparesis with Grade 3 power. He had no lymphadenopathy or hepatosplenomegaly. He was not immunocompromised magnetic resonance imaging Brain showed evidence of an irregular heterogeneous contrast-enhancing lesion in the left frontal lobe with surrounding edema and massive midline shift. The features were suggestive of high-grade glioma [Figure 1]. The patient underwent craniotomy and complete excision of the left frontal lobe mass lesion. Intraoperatively, the tumor was well defined with firm margins, moderate vascularity with a good plane of cleavage from the surrounding brain and could be excised en masse. The squash smears showed predominantly histiocytes with emperipolesis. The background showed numerous lymphocytes, histiocytes, occasional neutrophils, and atypical large cells. Scattered glial and neuronal cells are also shown [Figure 2]. Differential diagnosis of Rosai– Dorfman disease, histiocytic neoplasms, lymphoma, and melanoma were considered. Histopathological examination (hematoxylin and eosin-stained sections) showed brain tissue with an infiltrating tumor composed of scattered large cells showing large vesicular nuclei, prominent eosinophilic nucleoli resembling mononuclear Hodgkin’s cells, Reed Sternberg (RS) cells, and popcorn cells [Figure 3]. Atypical mitosis was noted with 7–10 mitotic figures per 10 high-powered fields. Other cells include lymphocytes and histiocytes with active phagocytosis. Large areas of necrosis and broad bands of fibrosis were seen. Immunohistochemical staining CD 45 was positive in all the hematopoietic cells and negative in the large tumor cells. The large cells expressed CD 15, CD30, nuclear positivity of PAX-5, and membranous positivity of CD79a. Ki-67 was 70%. GFAP, PAN CK, and HMB 45 were negative in the tumor cells [Figure 4]. Whole-body positron emission tomography (PET) computed tomography did not reveal any extra-cranial Hodgkin’s disease. Hence, this is a case of primary CNS HL in the left frontal lobe. Postoperatively, the patient made an uneventful recovery. Speech, power, right upper and lower limbs, and behavioral disturbance improved during the postoperative period. The patient was referred for further oncological treatment. The patient had improved completely at the review at two months.
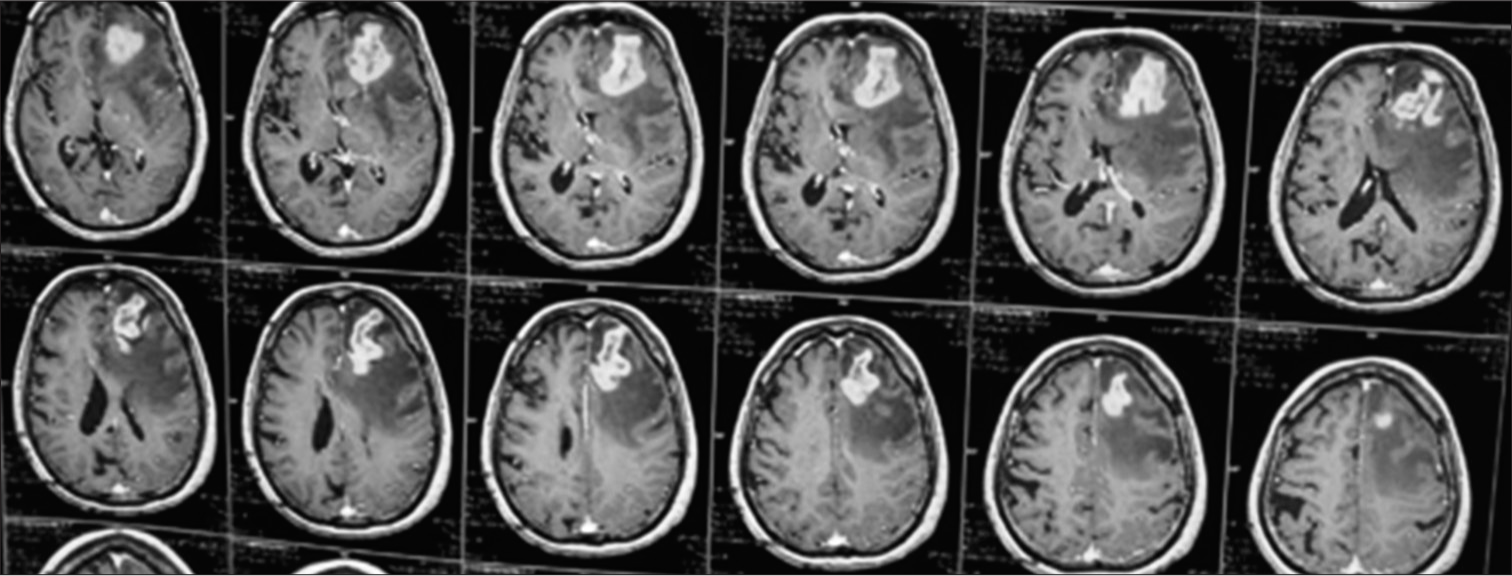
- MRI Brain (T1 contrast) showing irregular contrast enhancing mass lesion in the left frontal lobe with mass effect.
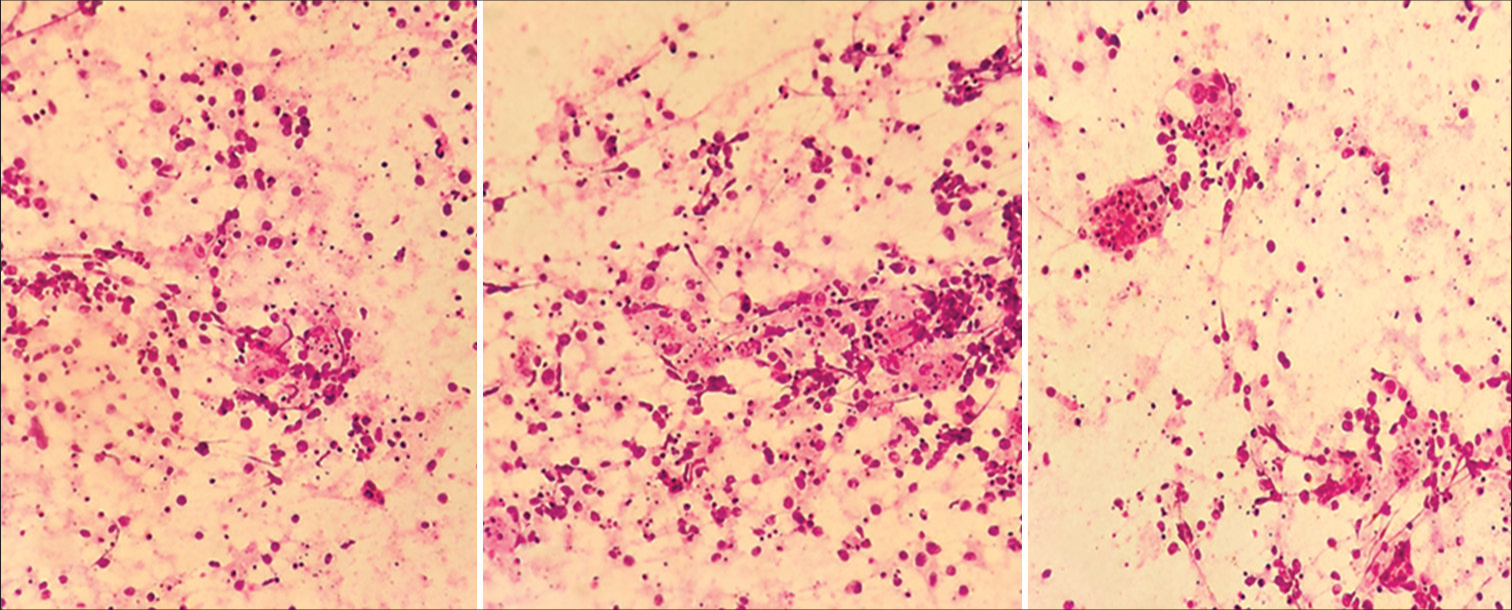
- Squash smears predominantly showing histiocytes with emperipolesis, H&E, 40X. H&E: Hematoxylin and eosin.
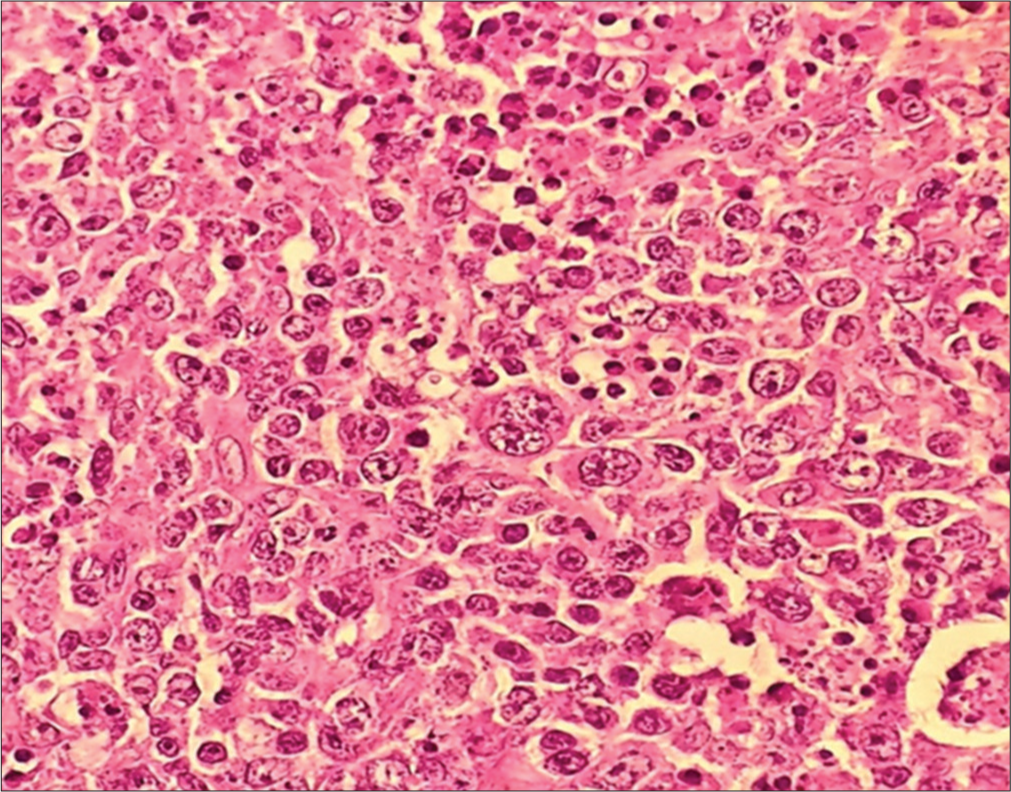
- Histopathological images of Hodgkin’s lymphoma with Reed-Sternberg cells, H&E, 40X. H&E: Hematoxylin and eosin.
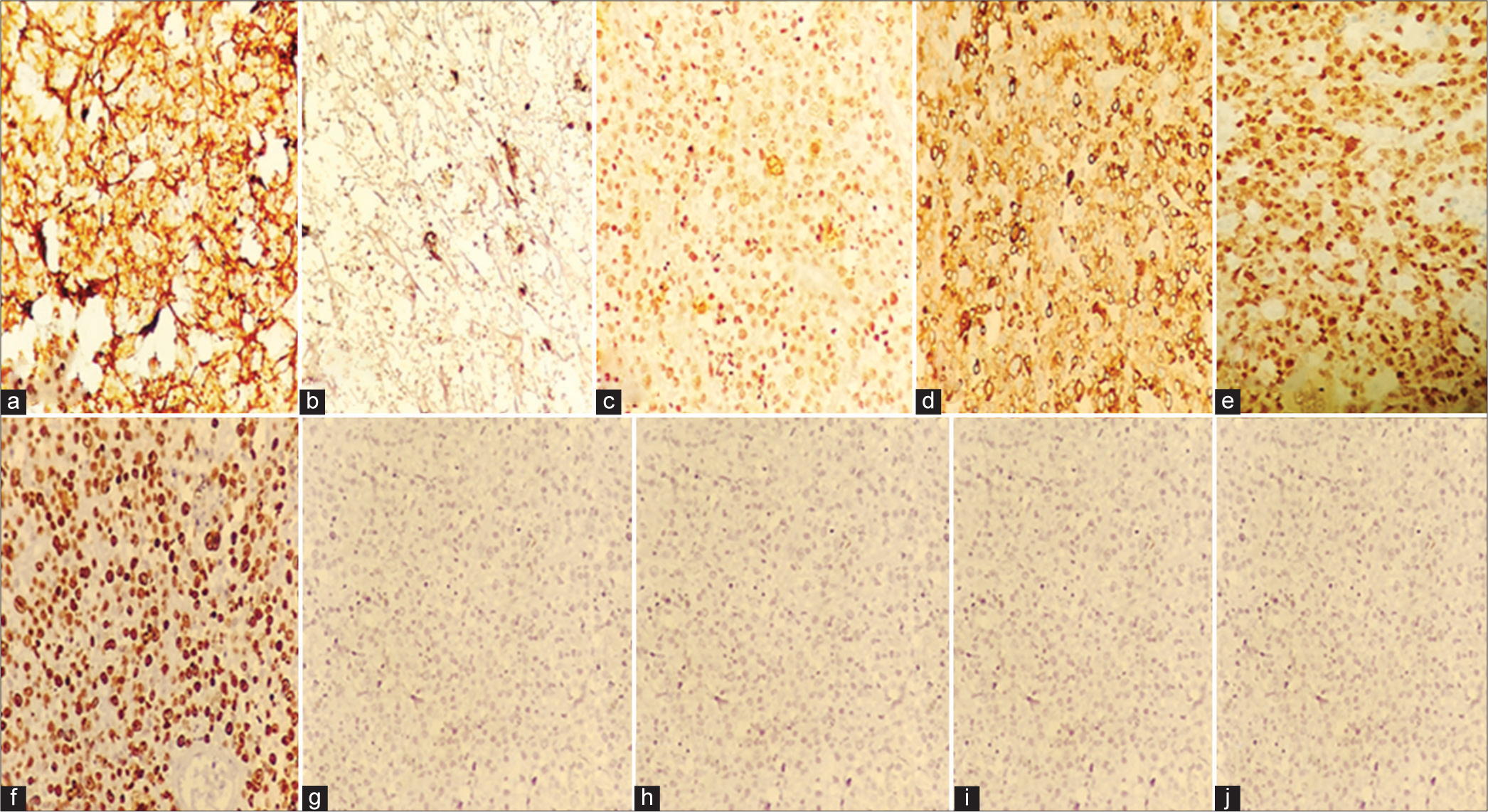
- (a) CD45 - Positive in all the hematopoietic elements, (b) CD15 - Cytoplasmic and membranous positivity of large cells, (c) CD30-Cytoplasmic and membranous positivity of large cells, (d) CD79a- Membranous positivity of large cells, (e) PAX-5- Weak nuclear positivity of large cells, (f) Ki-67: 70%, (g) HMB – 45: Negative in the tumor cells, (h) GFAP: Negative in the tumor cells, (i) Pan CK: Negative in the tumor cells, (j) EBV: Negative in the tumor cells.
DISCUSSION
Classical HL is primarily affecting the lymph nodes and CNS involvement is rare with HL, accounting for 0.2–0.5% of all cases.[5] The incidence of HL in CNS remains exceedingly rare in the literature with only 26 cases reported so far. The first 22 cases have been summarized by Cecyn et al. and five more cases (including the present case) have been added since then [Table 1].[7-11] There have been 18 males and nine females, showing a male preponderance. In 17 cases, the lesion was in the supratentorial compartment and the rest in the posterior cranial fossa. In four patients, the lesion was dura-based mimicking a meningioma. Twenty-two patients had normal immunological status and only five had some form of immune deficiency. The most common histological type has been reported as “Classical HL – not otherwise specified” (11 cases), followed by nodular sclerosis type (seven cases) and mixed cellularity (seven cases). The Epstein-Barr virus (EBV) status has been investigated only in 13 patients, and 10 patients have been reported positive for EBV. There has been no consensus on adjuvant therapy after surgery, with varying radiotherapy and/or chemotherapy regimens. The longest reported disease-free survival has been 10 years.
| Case | Author | Year | Age/sex | Immunocom promised | Location of the lesion | Type | EBV | Adjuvant treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Sparling et al.[9] | 1946 | 53/M | Unknown | Lt frontal lobe | Hodgkin’s sarcoma | Not investigated | None | Died 5 days after surgery |
| 2. | Scricker et al.[10] | 1955 | 45/M | Unknown | Rt temporal lobe | Non-sarcomatous Hodgkin’s granuloma | Not investigated | RT 1500 r | NED 36 months |
| 3. | Nagashima et al.[11] | 1980 | 60/M | Unknown | Falx cerebri | HL mixed cellularity type | Not investigated | None | Unknown |
| 4. | Bender and Mayernik[12] | 1986 | 34/M | Unknown | Rt frontal lobe and dura | HL nodular sclerosing type | Not investigated | RT+CT | NED 12 months |
| 5. | Doorly et al.[13] | 1987 | 51/M | No | Lt cerebellum | HL mixed cellularity type | Not investigated | RT 30 Gy | NED 12 months |
| 6. | Ashby et al.[14] | 1988 | 62/M | No | Rt frontoparietal region | HL nodular sclerosing type | Not investigated | RT 40Gy+IT CT | NED 14 months |
| 7. | Sickler et al.[15] | 1990 | 84/M | No | Rt parietooccipital region | Classical HL NOS | Not investigated | RT 35cGy | NED 8 months |
| 8. | Clark et al.[16] | 1992 | 53/F | Unknown | Rt cerebellum | HL nodular sclerosing type | Not investigated | RT 45 Gy | NED 6 months |
| 9. | Klein et al.[17] | 1999 | 54/M | No | Rt occipital lobe | HL nodular sclerosing type | Positive | RT 36 Gy+CT | NED 16 months |
| 10. | Blagi et al.[18] | 2000 | 52/M | No | Lt temporoparietal region | HL nodular sclerosing type | Negative | RT 30 Gy+5 Gy boost | NED 21 months |
| 11. | Johnson et al.[19] | 2000 | 55/F | No | Inferior aspect of tentorium cerebelli | HL nodular sclerosing type | Positive | RT 36 Gy+14 Gy boost | NED 8 months |
| 12. | de Castro et al. [20] | 2007 | 63?M | No | Lt frontoparietal region; Lt cerebellar hemisphere | Classical HL NOS | Positive | RT 40 Gy | Unknown |
| 13. | Hwang et al.[21] | 2007 | 64/F | No | Lt cerebellar hemisphere | HL mixed cellularity type | Not investigated | RT 30 Gy+6 Gy boost | NED 16 months |
| 14. | Gerstner et al.[22] | 2008 | 58/F | No | Unknown | Classical HL NOS | Positive | RT 35 Gy | NED 90.3 months |
| 15. | Gerstner et al. [22] | 2008 | 60/F | No | Cavernous sinus, Meckel’s cave, Lt mesial temporal lobe | HL nodular sclerosing type | Negative | RT | NED 1 month |
| 16. | Foo et al.[23] | 2011 | 58/M | No | Lt temporal lobe | Classical HL NOS | Positive | RT 45 GY+CT | Recurrence at 14 months |
| 17. | Kresak et al.[24] | 2013 | 70/M | Known COPD . H/o sq cell ca, HIV-ve | Lt cerebellum | Classical HL NOS | Not investigated | RT | NED 10 years |
| 18. | Kresak et al.[24] | 2013 | 72/F | No | Cerebellum | Classical HL NOS | Positive | RT | NED 6 months |
| 19. | Henkenberens et al.[25] | 2014 | 47/M | Known MS 20 years on azathioprine | Cerebellum | HL mixed cellularity type | Positive | RT 20 Gy+CT (BEACOPP) | NED 9 months |
| 20. | Mercadal et al.[26] | 2015 | 59/M | Known ulcerative colitis on azathioprine | Rt thalamus, midbrain | Classical HL NOS | Positive | CT (methotrexate+ cytosine arabinoside) | NED 65 months |
| 21. | Szelernej et al.[27] | 2016 | 47/F | Known dermatomyositis on methotrexate | Lt parietal lobe | Classical HL NOS | Not investigated | RT 30 Gy+6 Gy boost | NED 110 months |
| 22. | Cecyn et al.[7] | 2017 | 46/M | No | Rt frontal lobe | Classical HL NOS | Negative | RT 30 Gy+CT (ABVD) | NED 87 months died due to unrelated cause |
| 23. | Godbe et al.[8] | 2019 | 82/F | Rheumatoid arthritis, unknown skin cancer, chroic kidney disease | Lt parietooccipital region | HL mixed cellularity type | Positive | RT | Not known |
| 24. | Fu et al.[28] | 2021 | 60/M | No | Cerebellum | HL mixed cellularity type | Positive | CT (Methotrexate, cytosine arabinoside, and sintilimab) | NED 58 months |
| 25. | Alfaseh et al. [29] | 2019 | 38/M | No | Cerebellum | HL mixed cellularity type | Not investigated | RT 40 Gy+10 Gy boost+CT (ABVD) | NED 7 years |
| 26. | Wallizada et al.[30] | 2024 | 33/F | No | Dural based left sphenoid wing lesion | Classical HL NOS | Not investigated | CT (Multiple drug combinations) | NED 4 months |
| 27. | Present case | 2024 | 76/M | No | Left frontal lobe | Classical HL NOS | Not investigated | CT | NED 2 months |
Table adopted from: Godbe KN, Guilliams EL, Benko MJ, Grider DJ, Stump MS. primary central nervous system Hodgkin lymphoma versus lymphoproliferative disorder in an asymptomatic immunocompromised patient – a case report and review of the current literature. 2019 J. Blood Lymph 9: 250. And updated. NED: No evidence of disease, NOS: Not otherwise specified, HIV: Human immunodeficiency virus, CNS: Central nervous system, HL: Hodgkin’s lymphoma, CT: Computed tomography, Lt: Left, Rt: Right, RT: Radiotherapy, MS: multiple sclerosis, COPD: Chronic obstructive pulmonary disease, BEACOPP: Bleomycin sulfate, etoposide phosphate, doxorubicin hydrochloride (Adriamycin), cyclophosphamide, vincristine sulfate (Oncovin), procarbazine hydrochloride, and prednisolone, ABVD: Adriamycin, bleomycin sulfate, vinblastine sulfate, dacarbazine.
The primary CNS-HL is exceedingly rare and it is of interest to note that primary CNS-HL has not been included in the primary CNS (hematolymphoid tumors) lymphomas in the latest WHO Classification of tumors of the CNS (5th Edition). Immune compromised state, if present, may contribute to the development of primary CNS HL, though in the available literature, the majority of the patients had no evidence of immune deficiency. There has been male sex preponderance in the literature. The role of EBV has been of interest of late. The presence of EBV in the RS cells seems to correlate with the increased Treg cells and may contribute to the development of this tumor.[9] However, further research is needed in this regard. This may lead to better modalities of treatment, like targeting the Treg cells. About 82% of patients with classical HL have PD-L on the surface of tumor cells and EBV infection seems to increase the expression of PD-L on the RS cells.[9] It is of interest to note that anti-programmed cell death protein antibodies (anti-PD 1) have been used in the treatment of refractory HL. In the future anti-PD 1 antibody, like sintilimab, may form the first-line drug treatment in HL due to its relative safety compared to the potentially toxic first-line drugs used at present.
The present patient was a 76-year-old non-immunocompromised man presented with features of a rapidly growing malignant neoplasm in the left frontal lobe with imaging features suggestive of a high-grade parenchymal tumor possibly glioma. The histopathology and immunohistochemistry clinched the diagnosis of HL. We could not do the EBV DNA testing and PD-L staining which could have been very useful. The whole body PET did not reveal any focus elsewhere in the body. Hence, this is a rare case of primary CNS-HL.
CONCLUSION
Primary CNS-HL should be kept in the differential diagnosis of intracranial malignant tumours, especially in old men, with immunocompromised state. EBV-DNA testing and PD-L staining should be performed in all cases of suspected HL to establish the diagnosis and this will also help in planning adjuvant chemotherapy.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consents.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Central nervous system lymphoma: Characteristic findings on traditional and advanced imaging. Am J Neuroradiol. 2011;32:984-92.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging features of intracranial primary CNS lymphoma in immune competent patients. Cancer Imaging. 2014;14:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary CNS lymphomas: Challenges in diagnosis and monitoring. Biomed Res Int. 2018;2018:3606970.
- [CrossRef] [PubMed] [Google Scholar]
- Myriad of MR imaging phenotypes of primary central nervous system lymphoma in a cohort of immunocompetent Indian patient population. Indian J Radiol Imaging. 2018;28:296-304.
- [CrossRef] [PubMed] [Google Scholar]
- Central nervous system Hodgkin lymphoma: Case report and review of the literature. J Neurooncol. 2011;102:329-34.
- [CrossRef] [PubMed] [Google Scholar]
- Primary central nervous system involvement in classical Hodgkin's lymphoma: Case report and review of the literature. J Blood Lymph. 2017;8:196.
- [Google Scholar]
- Primary central nervous system Hodgkin lymphoma versus lymphoproliferative disorder in an asymptomatic immunocompromised patient-a case report and review of the current literature. J Blood Lymph. 2019;9:250.
- [CrossRef] [Google Scholar]
- Primary intracerebral Hodgkin's disease. Cancer. 1955;8:629-33.
- [CrossRef] [PubMed] [Google Scholar]
- Primary Hodgkin's disease of the falx cerebri. Acta Neuropathol. 1980;51:161-3.
- [CrossRef] [PubMed] [Google Scholar]
- Hodgkin's disease presenting with isolated craniospinal involvement. Cancer. 1986;58:1745-8.
- [CrossRef] [PubMed] [Google Scholar]
- Primary intracerebral Hodgkin's lymphoma. J Neurol Neurosurg Psychiatry. 1987;50:1048-50.
- [CrossRef] [PubMed] [Google Scholar]
- Primary intracranial Hodgkin's disease. A case report and discussion. Am J Surg Pathol. 1988;12:294-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary intracerebral Hodgkin's disease: A case report. Clin Neuropathol. 1990;9:143-7.
- [Google Scholar]
- Primary intracranial Hodgkin's lymphoma without dural attachment: Case report. J Neurosurg. 1992;76:692-5.
- [CrossRef] [PubMed] [Google Scholar]
- Primary intracerebral Hodgkin's disease: Report of a case with epstein-barr virus association and review of the literature. Am J Surg Pathol. 1999;23:477-81.
- [CrossRef] [PubMed] [Google Scholar]
- Primary Hodgkin's disease of the CNS in an immunocompetent patient: A case study and review of the literature. Neurooncology. 2000;2:239-43.
- [CrossRef] [Google Scholar]
- Primary intracerebral Hodgkin's disease mimicking meningioma: Case report. Neurosurgery. 2000;47:454-7.
- [CrossRef] [PubMed] [Google Scholar]
- Primary intracerebral Hodgkin lymphoma. Br J Haematol. 2007;138:562.
- [CrossRef] [PubMed] [Google Scholar]
- Primary intracerebral Hodgkin lymphoma with recurrence. Clin Neuropathol. 2011;30:75-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary EBV-positive Hodgkin's lymphoma of the CNS under azathioprine treatment. Strahlenther Onkol. 2014;190:847-52.
- [CrossRef] [PubMed] [Google Scholar]
- Long term remission of a primary intracerebral Hodgkin lymphoma in a patient previously treated with azathioprine. J Integr Oncol. 2015;4:144.
- [CrossRef] [Google Scholar]
- Treatment and long-term follow-up of primary CNS classical Hodgkin's lymphoma-A case report and review of the literature. Interdisciplin Neurosurg. 2017;9:30-3.
- [CrossRef] [Google Scholar]
- Primary central nervous system Hodgkin's lymphoma: A case report. J Int Med Res. 2021;49:300060521999533.
- [CrossRef] [PubMed] [Google Scholar]
- Primary central nervous system Hodgkin Lymphoma: A case discussion and a hypothesis on the etiology. Avicenna J Med. 2019;9:28-31.
- [CrossRef] [PubMed] [Google Scholar]
- Classic central nervous system Hodgkin lymphoma masquerading as left sphenoid wing meningioma-A case report. Int J Surg Case Rep. 2024;116:109439.
- [CrossRef] [PubMed] [Google Scholar]






