Translate this page into:
Posture-dependent aphasia: Focal cortical dysfunction in the sinking scalp flap syndrome
Address for correspondence: Dr. Prasad Krishnan, Department of Neurosurgery, National Neurosciences Centre, Peerless Hospital Complex, 2nd Floor, 360, Panchasayar, Garia, Kolkata - 700 094, West Bengal, India. E-mail: prasad.krishnan@rediffmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Decompressive craniotomies are being increasingly used in the treatment of raised intracranial pressure due to a variety of reasons like large infarcts, hypertensive hemorrhages and contusions. Though effective in decreasing raised intracranial pressure, they have certain complications like the sinking scalp flap syndrome that is caused by cortical dysfunction of the area below the craniotomy which is exposed to the effects of atmospheric pressure. We describe a 60-year-old patient who underwent decompressive craniotomy for acute subdural hematoma and after an initial uneventful postoperative period developed incontinence, irrelevant verbalization and ataxia. He was found to have hydrocephalus and underwent a ventriculo-peritoneal shunt with resolution of his symptoms. Three weeks later his flap had sunk in deeply and the skin was non-pinchable and he was noted to have headaches, vomiting and retching when he sat up. In addition he became aphasic when seated and the symptoms subsided on lying down. A diagnosis of focal cortical dysfunction due to sinking scalp flap syndrome was made. We highlight the incidence and pathophysiology of this unusual complication of decompressive craniotomy and stress the need to be aware of this entity particularly in patients who do not show an initial improvement after decompressive craniotomy as the cause of their poor neurological status may not be explained by any other mechanism.
Keywords
Aphasia
decompressive craniotomy
hydrocephalus
sinking scalp flap syndrome
syndrome of the trephined
Introduction
Yamamura and Makino (1977) first described the sinking scalp flap syndrome following decompressive craniotomy.[1] It consisted of “objective neurological deficits” that could be explained by dysfunction of the cortex below the concave scalp flap and which showed improvement following cranioplasty. On the other hand, syndrome of the trephined which was described by Grant and Norcross in 1939 consisted of more protean features of headache, seizures, mood swings, memory and behavioral disturbances.[2] However, the term “motor trephine syndrome” has been used by some authors to highlight the delayed motor deficits following decompressive craniotomy that reverse with cranioplasty and this term has been used as an eponym of sinking scalp flap syndrome.[3]
The incidence of syndrome of the trephined was reported to be 10 of 38 (26%) patients by Stiver while following up those who underwent cranioplasty in a series of 170 patients who had undergone decompressive craniotomy for traumatic brain injury.[4] However, most of the literature obtained is in the nature of isolated case reports and does not throw light on the incidence rate of this complication. We have ourselves encountered rapid documentable improvement in motor function in only 2 patients out of 107 who underwent cranioplasty and including this patient would venture to estimate the rate of this complication to be 3 out of 107, i.e., 2.8%.
Clinical presentation
A 60-year-old male patient underwent decompressive craniectomy for a left-sided post-traumatic fronto-temporal acute subdural hematoma and had a satisfactory postoperative recovery. Four months later he presented with irrelevant verbalization, urinary incontinence and ataxia. His decompressive craniectomy site which was previously lax began to bulge. Computed tomography (CT) scan of brain revealed communicating hydrocephalus. He underwent a right ventriculoperitoneal (VP) shunt with a slit and spring valve and had an uneventful postoperative period. He became oriented, continent and was independently ambulant. Three weeks later he was again admitted with complaints of headache, retching (and occasional vomiting) whenever he sat up. His relatives had noted that he stopped talking when he was made to sit or stand but regained speech within minutes of lying down. The patient could read and comprehend in the upright and supine positions. Repetition and writing were impaired in the upright position. He was however able to write his name and address while lying down. There was no posture-related motor dysfunction. There was no evidence to suggest seizures, postural hypotension or syncope. His skin flap was deeply sunken [Figure 1a and b]. An electroencephalogram was done that showed diffuse slowing but no epileptiform activity. CT scan of brain showed a concave depression of the scalp and underlying brain at the site of the craniotomy with effacement of cortical sulci at this site. The shunt tip was in situ in the right frontal horn and midline shift to the right was noted [Figure 1c]. By exclusion of other pathologies, sinking scalp flap syndrome with focal cortical dysfunction of the speech area was diagnosed and the patient underwent cranioplasty. He subsequently was verbalizing normally in erect and supine positions. Postoperative CT scan also showed complete expansion of the brain [Figure 1d].
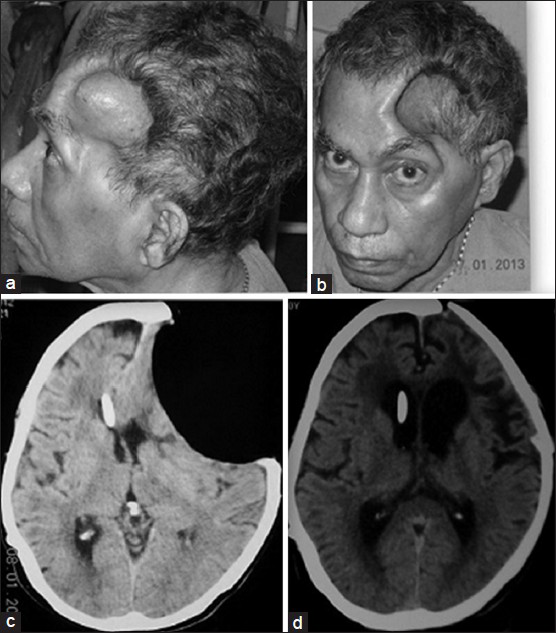
- (a and b) Deeply punched out concavity is seen in the left frontotemporal region with the patient seated with the skin drawn in tightly over the margins of the craniotomy defect. (c) Axial CT scan of brain showing sunken skin flap with pressure on the underlying brain, obliteration of sulcal patterns at the concavity, midline shift and shunt in situ in the contralateral ventricle. (d) Axial CT scan shows the brain has completely expanded with appearance of normal sulcal patterns and restoration of the midline
Discussion
Decompressive craniotomy coverts the closed box of the cranium into an “open box.” Once the raised intracranial pressure (for which the craniectomy is performed) abates, the atmospheric pressure begins to push the skin with underlying brain, with no skull bone to protect it, to the opposite side. Compression of the cortical surface by the scalp flap, alteration in cerebral blood flow, impedance of cortical venous return and abnormal cerebrospinal fluid (CSF) circulation have all been implicated as causative factors in sinking scalp flap syndrome.[345] The inward pressure of the atmosphere is resisted by two mechanisms—the elastance of the brain (that resists deformation) and the innate tendency of the ventricular CSF to keep the brain expanded—and is the reason why this syndrome is uncommonly reported. Indeed it has been reported that parenchymal injury, presumably with tissue loss and increased edema causing decreased capacity to resist atmospheric pressure, is a positive predictive factor for the development of this syndrome.[4] CSF drainage, too, further increases the pressure gradient between the brain and the atmosphere[67] and this may in turn increase the local pressure on the underlying cortex or cause diminution of blood flow therein. Imaging often shows a deeply concave punched-out appearance on CT scan as in our patient.
In our patient the intracranial pressure was already low following the VP shunt. Subsequently when he used to sit up, decreased cerebral venous pressure probably led to a further decrease in resistance of the brain to atmospheric pressure with further focal cortical compression. After excluding causes like seizures, syncope, postural hypotension we felt that this posture-dependent aphasia was due to left frontal lobe dysfunction due to sinking scalp flap and proceeded for cranioplasty following which he improved. There is only one report previously in the literature by Nakamura et al.[8] specifically outlining speech dysfunction in a patient (who too had undergone craniectomy and VP shunt) with change to erect posture. This too reversed after restoration to supine position. Relation between posture and other non-aphasic manifestations of neurological dysfunction in such patients is mentioned in other reports by Joseph et al.[3] and Bijlenga et al.[9] as well. As our patient had no complaints after cranioplasty even with the shunt in situ we do not think that CSF over-drainage in the erect posture was responsible for the findings and would choose to hold posture-dependent change in brain resistance responsible for the same.
Conclusion
Finding a concave scalp flap after decompressive craniotomy, particularly if the patient has been shunted, is not unusual. Aphasia precipitated by adoption of erect posture was the uncommon and easily identifiable neurological finding in this patient that drew our attention to the fact that he might be having the “sinking scalp flap syndrome.”
If deterioration occurs after an initial improvement sinking scalp flap syndrome must be kept in mind as a potential cause. While this syndrome is very difficult to pick up in cases that have not shown an initial improvement, the condition of the scalp flap and CT scan picture must alert the physician that lack of improvement may be due to focal cortical dysfunction induced by atmospheric pressure which may be reversed by cranioplasty once other causes are excluded.
Acknowledgement
We are grateful to the family and his relatives for allowing us to use the clinical and radiological images.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Neurological deficits in the presence of the sinking scalp flap following decompressive craniectomy. Neurol Med Chir (Tokyo). 1977;17:43-53.
- [Google Scholar]
- Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. 2009;26:E7.
- [Google Scholar]
- The impact of cranioplasty on neurological function. Br J Neurosurg. 2013;27:636-41.
- [Google Scholar]
- Sinking skin flap syndrome after craniectomy in a patient who previously underwent a ventriculoperitoneal shunt. Korean J Neurotrauma. 2012;8:149-52.
- [Google Scholar]
- “Syndrome of the sinking skin-flap” secondary to the ventriculoperitoneal shunt after craniectomy. J Korean Neurosurg Soc. 2008;43:51-3.
- [Google Scholar]
- Rapid neurological alteration associated with concave deformity of the skin flap in a craniectomized patient. Case report. Neurol Med Chir (Tokyo). 1980;20:89-93.
- [Google Scholar]
- Orthostatic mesodiencephalic dysfunction after decompressive craniectomy. J Neurol Neurosurg Psychiatry. 2007;78:430-3.
- [Google Scholar]






