Translate this page into:
Posterior reversible encephalopathy syndrome in immunocompromised children – A single-center study from South India
*Corresponding author: Sudeep Gaddam, Department of Pediatric Hematology Oncology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India. sudeepgaddam2@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Gaddam S, Kodandapani R, Mani N, Jayaraman D, Nikitha Abirami B. Posterior reversible encephalopathy syndrome in immunocompromised children – A single-center study from South India. J Neurosci Rural Pract. 2024;15:365-9. doi: 10.25259/JNRP_390_2023
Abstract
This study describes the profile of children diagnosed with posterior reversible encephalopathy syndrome (PRES) in the pediatric hematology oncology unit and highlights the clinical features of PRES in immunosuppressed children. This retrospective study included 10 children diagnosed with PRES with a mean age of 6.8 years. Acute lymphoblastic leukemia was the most common primary diagnosis followed by post-hematopoietic stem cell transplant patients. Most cases of PRES occurred within one month of treatment initiation. Hypertension was noted in all at the time of diagnosis. Neuroimaging revealed bilateral lesions with parietal and occipital lobe involvement being the most common. All patients received corticosteroids as part of treatment for primary diagnosis. Controlling blood pressure was critical in managing PRES. Consideration of PRES as a clinical possibility in pediatric hematology oncology unit in children presenting with symptoms such as headache, seizures, and visual disturbances will aid in early diagnosis after ruling out other causes of these symptoms.
Keywords
Posterior reversible encephalopathy syndrome
Pediatric hematology-oncology unit
Hypertension
Hematopoietic stem cell transplantation
Childhood cancer
Immunocompromised host
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is a clinicoradiological diagnosis with seizures, headache, visual disturbances, and altered sensorium as a predominant clinical feature. Etiopathogenesis of PRES is not clear.[1] Hypertension is a substantial contributing factor in the development of PRES.[2] Neuroimaging shows typical transient lesions involving parietal, occipital, and frontal lobes.[3,4] Early diagnosis and symptomatic treatment will result in the complete resolution of symptoms without any long-term sequelae. The PRES has been described in the pediatric population with leukemia and post-hematopoietic stem cell transplant (HSCT), but so far, information consists of case reports or small series and only a few studies. In this case series, we herein describe the profile of children diagnosed with PRES in a pediatric hematology oncology unit.
MATERIALS AND METHODS
A retrospective study was conducted between November 1, 2017, and December 1, 2022, in our department of pediatric hematology oncology. Institutional ethics committee approval was obtained. All children with a radiological diagnosis of PRES under 18 years were enrolled. Our pediatric hematology oncology unit has nearly 100 newly diagnosed childhood cancer cases each year and 15 bone marrow transplants per year. After obtaining informed consent, the following parameters were collected from the hospital records: Primary diagnosis of the child, phase of treatment, drugs used before the diagnosis of PRES, blood pressure charts, PRES-symptomatology, radiological investigations, treatment, and outcome on follow-up. Mean systolic and/or mean diastolic blood pressure ≥95th percentile was considered hypertension. Magnetic resonance imaging (MRI) of the brain with bilateral white matter edema in the posterior cerebral hemispheres and cerebellum was considered a typical finding, and other radiological sites were involved as atypical presentations of PRES. Data is presented in interquartile ranges, mean, medians, frequencies or percentages.
RESULTS
A total of 10 children were diagnosed to have PRES. The mean age was 6.8 years (range, 4–11 years). Male-to-female ratio of 1.5:1. In this study, 8 out of 10 children developed PRES within one month of treatment for their primary diagnosis. The most common primary diagnosis was acute lymphoblastic leukemia (n = 5) followed by post-HSCT patients (n = 2), Burkitt’s lymphoma (n = 1), intracranial nongerminomatous germ cell tumor (n = 1), and metastatic Ewing sarcoma (n = 1). Most of the patients were in the initial phase of treatment where the onset of PRES was within one month of chemotherapy whereas two children with post-HSCT (n = 2) developed PRES on day 40 and day 50 post-HSCT for Fanconi anemia and dyskeratosis congenita, respectively. The 9 out of 10 patients received corticosteroids; two had received tacrolimus before the onset of PRES; and all patients had hypertension at the time of diagnosis. Seizures (n = 6) were the most common clinical feature followed by visual disturbances (n = 5), headache (n = 4), altered sensorium (n = 2), and pyramidal weakness (n = 1). Among the patients with visual disturbances, one had cortical blindness. Symptoms at presentation are summarized in the Figure 1. All 10 patients had bilateral lesions, 70% had occipital and parietal lobe involvement, and in two patients where corpus callosum, basal ganglia, thalamus, and hippocampal regions were involved as atypical PRES. The characteristics of patients with PRES and the site of radiological involvement on MRI are summarized in Table 1. All patients required treatment in the pediatric intensive care unit. In our study, 6 out of 10 required mechanical ventilation, anti-seizure medication was required for seven patients, oral nifedipine was used in all 10 patients whereas two required hypertonic saline infusion, one required prazosin, and enalapril was required in four patients to control hypertension. All patients recovered completely well after the episode of PRES. Six got repeat MRIs on follow-up, which showed complete resolution of findings of PRES with no recurrence of seizures in anyone. Blood pressure remained within normal limits for all.
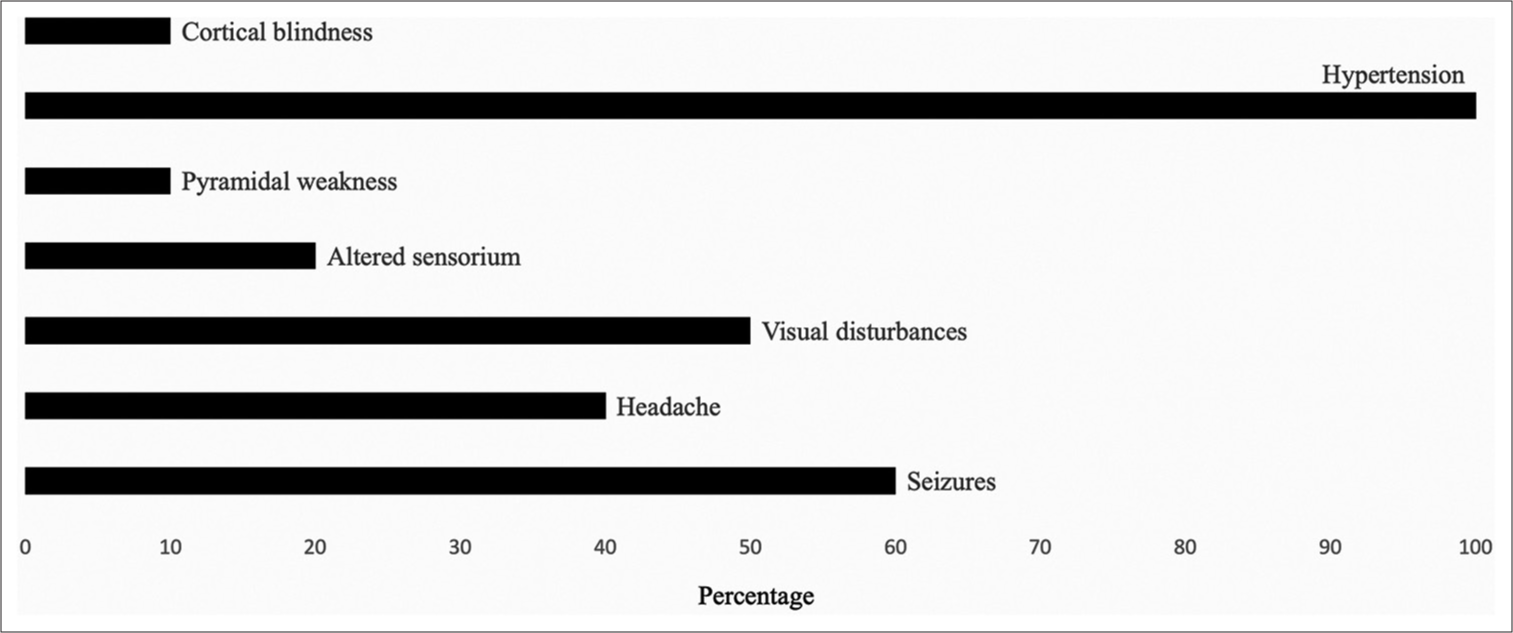
- Symptoms at presentation.
| Diagnosis | Gender | Age in years | Period of treatment to onset of PRES | Treatment underwent so far | Site of radiological involvement | |
|---|---|---|---|---|---|---|
| 1. | B-cell acute lymphoblastic leukemia | Male | 6 | 5 days | Dexamethasone, Vincristine, Pegylated Asparaginase, and Doxorubicin | Fronto-parieto-occipital |
| 2. | T cell acute lymphoblastic leukemia | Male | 6 | 5 days | Dexamethasone, Vincristine, and Pegylated Asparaginase, | Parieto-occipital |
| 3. | Burkitt lymphoma | Male | 6 | 3 days | Prednisolone, Cyclophosphamide, and Vincristine | Occipito-fronto-parietal |
| 4. | Intracranial non-germinomatous germ cell tumor | Female | 5 | 5 days | Carboplatin and Etoposide | Parieto-occipital-cerebellar |
| 5. | Metastatic Ewing sarcoma | Male | 4 | 7 days | Vincristine, Doxorubicin, Cyclophosphamide, Ifosfamide, and Etoposide | Basal ganglia-corpus callosum-cerebral white matter |
| 6. | B-cell Acute Lymphoblastic Leukemia | Female | 10 | 14 days | Dexamethasone, Vincristine, Pegylated Asparaginase, and Doxorubicin | Parieto-occipital |
| 7. | Fanconi Anemia | Female | 11 | 40 days | Post-Hematopoietic Stem Cell Transplant – Tacrolimus, and Prednisolone | Cerebral-cerebellar-left Basal ganglia |
| 8. | Dyskeratosis congenita | Female | 5 | 50 days | Post-Hematopoietic Stem Cell Transplant – Tacrolimus and Prednisolone | Parieto-occipital |
| 9. | B cell Acute Lymphoblastic Leukemia | Male | 7 | 4 days | Prednisolone | Fronto-parieto-occipital with cerebral and cerebellar atrophy |
| 10. | T-cell Acute Lymphoblastic Leukemia | Male | 8 | 16 days | Dexamethasone, Vincristine, Pegylated Asparaginase, Daunorubicin, and Methotrexate | Fronto-parieto-occipital |
PRES: Posterior reversible encephalopathy syndrome
DISCUSSION
A diagnosis of PRES was made in 10 cases over a five-year period in immunosuppressed children. Central nervous system leukemia, meningoencephalitis, methotrexate-induced leukoencephalopathy, and changes due to metabolic disturbances were among the differential diagnosis of PRES due to the immunocompromised state in cancer and hematological stem cell transplant.[5]
In our study, we looked into comorbidities, underlying primary diagnosis, chemotherapeutic use, and post-hematopoietic stem cell transplantation as potential risk factors for the emergence of PRES. In a five-year retrospective study by Sommers et al. to investigate the incidence of PRES in 473 children with hematologic malignancy or post-allogeneic hematopoietic cell transplantation found 14 patients diagnosed with PRES resulting in an incidence rate of 5.9 cases/1000 people/year, and they also found a significant association between hematopoietic cell transplantation and the development of PRES.[3]
In our study along with leukemia, we had other solid tumors and post-HSCT patients.[3,4] Hun et al. found that mortality among PRES with HSCT was two times that of oncologic or hematologic disease where 65% of patients were receiving chemotherapy of which 44.8% were in induction regimen and 86.5% were on corticosteroids before the onset of PRES.[6]
Corticosteroids were used in 90% whereas 20% were on tacrolimus, and the majority were leukemia followed by post-HSCT in our study. Although in general, the etiopathogenesis of PRES is unclear; however, it has been proposed that several chemotherapy drugs directly destroy the vascular endothelium. In addition, because of the increased risk of steroid-related hypertension, corticosteroids may indirectly contribute to the emergence of PRES. Renal impairment may also contribute to hypertension during therapy for pediatric hematology oncology conditions. All our patients, who developed PRES, had their renal function tests within normal limits. The most crucial factor is the presence of hypertension, neither the disease nor treatment.[7] This suggests that proper blood pressure control is crucial when treating immunosuppressed children. All the patients in our study had documented hypertension at the time of diagnosis of PRES.[6]
The PRES resulted from treatment that occurred in children with acute lymphoblastic leukemia. Like our findings, all other investigators identified ALL induction chemotherapy as the primary risk factor for developing PRES in young patients with leukemia.[8] It is challenging to determine the specific chemotherapy agent that caused PRES in these children since many chemotherapeutic medications used to treat leukemia including vincristine, L-asparaginase, intrathecal methotrexate, and cytarabine have been connected to PRES; however, no specific medication or treatment plan has been linked to PRES in leukemia.[8,9]
The PRES is characterized by a combination of symptoms including seizures, altered mental status, visual disturbances, and headaches. Our profile of PRES had clinical features such as seizures, headaches, altered sensorium, and cortical blindness that are identical to PRES in the pediatric group with and without cancer.[1,2,10]
The PRES is seen commonly in the posterior circulation involving the occipital and parietal lobes. Atypical PRES has features of hemorrhage, enhancement, restricted diffusion, and brainstem involvement as in Figure 2. According to radiological data, typical PRES was more prevalent than atypical PRES. In Egypt, Hafez et al. found that atypical PRES, status epilepticus at presentation, and motor dysfunction with frontal lobe and thalamic involvement had unfavorable outcomes.[10] In our study, two-thirds had typical PRES most commonly involving parieto-occipital lobes followed by frontal, basal ganglia, cerebellum, and corpus callosum.[4]
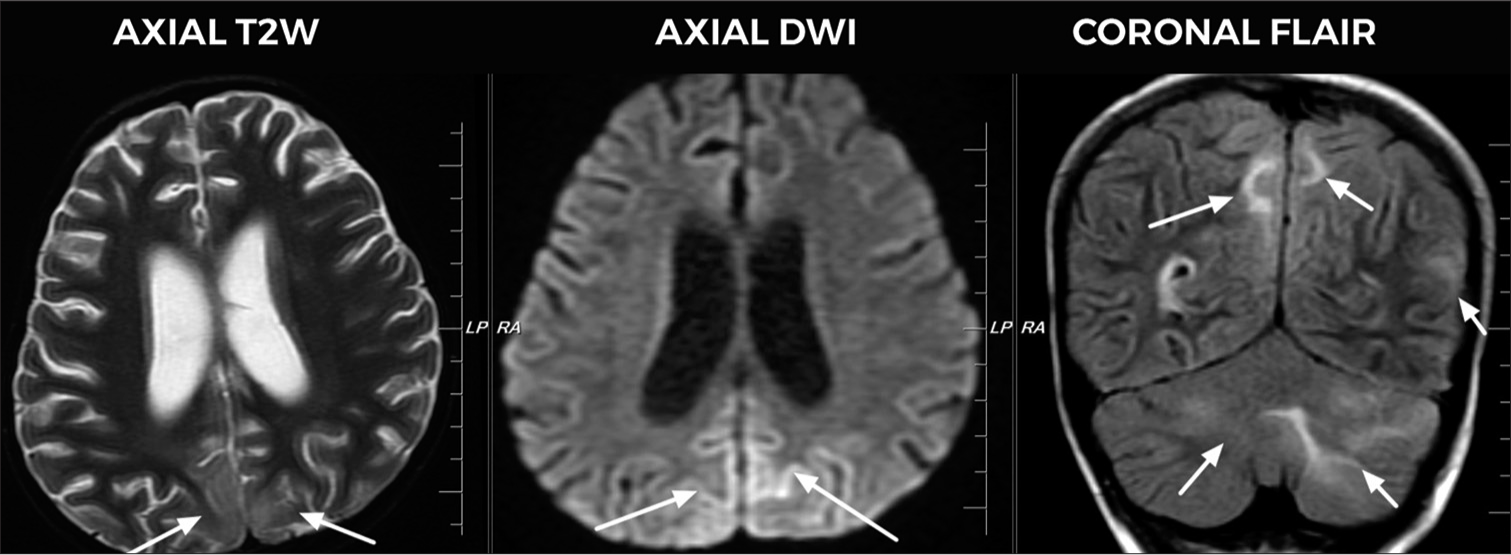
- Five-year-old girl with intracranial non-germinomatous germ cell tumor of suprasellar region who presented with visual disturbances and altered sensorium, magnetic resonance imaging image shows bilateral symmetrical T2/fluid-attenuated inversion recovery hyperintensities (arrows) with subtle restricted diffusion is seen in bilateral parieto-occipital lobes and bilateral cerebellar hemispheres, suggestive of posterior reversible encephalopathy syndrome. In the coronal flair, bilateral optic nerve sheath complexes appear prominent with mild kinking of optic nerves, suggestive of raised intracranial tension.
Although all the patients required intensive care, no one died due to PRES, and no residual radiological findings were detected on follow-up. The neurological and radiological findings are transient as the term PRES. In our study, we did not notice any recurrence of PRES or long-term sequelae such as epilepsy or focal deficits on follow-up even after restarting the chemotherapy; hence, close monitoring of blood pressure and early symptomatic treatment of neurological manifestations is essential for preventing long-term sequelae.
The limitations of this study include a retrospective study where data was extracted from already recorded medical records and a single center with a small sample size limiting the generalizability of the study results.
CONCLUSION
The PRES is common in immunosuppressed children and triggered by hypertension, which may be episodic. Consideration of PRES as a clinical possibility in pediatric hematology oncology unit in children presenting with symptoms such as headache, seizures, and visual disturbances will aid in early diagnosis after ruling out other causes of these symptoms. It is important to exclude other relevant causes. Early identification and effective management of hypertension will prevent long-term sequelae.
Acknowledgments
None.
Ethical approval
The research/study was approved by the Institutional Review Board at the Institutional Ethics Committee at Sri Ramachandra Institute of Higher Education and Research, Chennai, India, number IEC-NI/22/JAN/81/09, dated 09.03.2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Etiology and clinical characteristics of pediatric non-neoplastic posterior reversible encephalopathy: Systematic review. Porto Biomed J. 2022;7:e147.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical features and prognostic analysis of posterior reversible encephalopathy syndrome in children. Int J Dev Neurosci. 2022;82:349-60.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior reversible encephalopathy syndrome: Incidence and clinical characteristics in children with cancer. J Pediatr Hematol Oncol. 2022;44:54-9.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior reversible encephalopathy syndrome in pediatric cancer: Clinical and radiologic findings. J Glob Oncol. 2018;4:1-8.
- [CrossRef] [Google Scholar]
- Posterior reversible encephalopathy syndrome in the setting of asparaginase-associated pancreatitis in 2 pediatric patients with acute leukemia. J Pediatr Hematol Oncol. 2022;44:E709-12.
- [CrossRef] [PubMed] [Google Scholar]
- Management and clinical outcome of posterior reversible encephalopathy syndrome in pediatric oncologic/hematologic diseases: A PRES subgroup analysis with a large sample size. Front Pediatr. 2021;9:678890.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior reversible encephalopathy syndrome in childhood cancer. Ann Oncol. 2011;22:472-8.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia: Clinical characteristics, risk factors, course, and outcome of disease. Pediatr Blood Cancer. 2019;66:e27594.
- [CrossRef] [PubMed] [Google Scholar]
- Posterior reversible encephalopathy syndrome after high dose cytarabine in pediatric acute myeloid leukemia. J Appl Hematol. 2021;12:118.
- [CrossRef] [Google Scholar]
- Patterns, risk factors and outcome predictors of posterior reversible encephalopathy syndrome in pediatric cancer patients. Leuk Lymphoma. 2021;62:462-8.
- [CrossRef] [PubMed] [Google Scholar]







